Is Air An Element Or Compound
Juapaving
Mar 14, 2025 · 6 min read
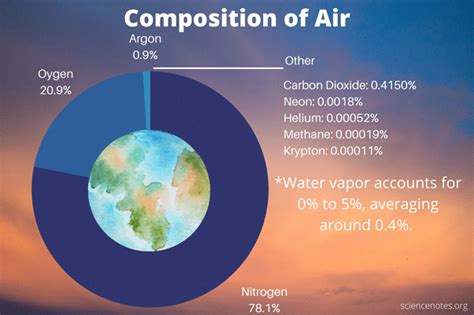
Table of Contents
Is Air an Element or a Compound? Unraveling the Composition of Our Atmosphere
The simple question, "Is air an element or a compound?" leads to a fascinating exploration of chemistry, physics, and the very essence of our atmosphere. While seemingly straightforward, the answer requires a deeper understanding of the fundamental differences between elements, compounds, and mixtures. This article will delve into the intricacies of air's composition, clarifying its classification and highlighting the crucial role each component plays in sustaining life on Earth.
Understanding the Basics: Elements, Compounds, and Mixtures
Before we tackle the air question, let's establish a clear understanding of the core concepts:
Elements:
Elements are fundamental substances that cannot be broken down into simpler substances by chemical means. They are made up of only one type of atom. Examples include oxygen (O), nitrogen (N), hydrogen (H), and iron (Fe). Each element is defined by its unique atomic number, representing the number of protons in its nucleus.
Compounds:
Compounds are substances formed when two or more elements chemically combine in fixed proportions. This combination involves a chemical reaction, resulting in a new substance with properties different from its constituent elements. For example, water (H₂O) is a compound formed from the combination of hydrogen and oxygen. The properties of water are distinctly different from those of hydrogen and oxygen gases. The chemical bonds within a compound are strong and require significant energy to break.
Mixtures:
Mixtures are combinations of two or more substances that are not chemically bonded. The components of a mixture retain their individual properties and can be separated by physical methods like filtration, distillation, or evaporation. Air is a prime example of a mixture.
The Composition of Air: A Gaseous Mixture
Air is a homogeneous mixture, meaning its composition is uniform throughout. It's not a compound because the gases that constitute air are not chemically bonded. Instead, they exist as individual molecules, intermingled and interacting through physical forces, not chemical bonds.
The primary components of air are:
- Nitrogen (N₂): Approximately 78% of the air's volume is nitrogen. While relatively inert, nitrogen plays a vital role in the nitrogen cycle, essential for plant growth and ultimately, the entire food chain.
- Oxygen (O₂): Oxygen constitutes about 21% of the air's volume. It is crucial for respiration in most living organisms, supporting cellular metabolism and energy production. Oxygen's highly reactive nature fuels combustion and numerous other chemical processes.
- Argon (Ar): This inert noble gas makes up about 0.93% of air. Its chemical inactivity prevents it from interfering with the other components of the atmosphere.
- Carbon Dioxide (CO₂): While present in a smaller percentage (around 0.04%), carbon dioxide plays a crucial role in the Earth's climate and in photosynthesis. It is a greenhouse gas, trapping heat and regulating the planet's temperature. Increases in atmospheric CO₂ levels are a significant concern related to climate change.
- Other Gases: Trace amounts of other gases, including neon (Ne), helium (He), methane (CH₄), krypton (Kr), hydrogen (H₂), and nitrous oxide (N₂O), are also present in the atmosphere. These gases, although in small quantities, can have significant environmental impacts. For instance, methane is a potent greenhouse gas, and nitrous oxide contributes to ozone depletion.
- Water Vapor (H₂O): The amount of water vapor in the air varies significantly depending on location and weather conditions. It's an important component of the water cycle and influences humidity and precipitation.
Why Air Isn't a Compound: A Closer Look
The key to understanding why air is not a compound lies in the absence of chemical bonds between its constituent gases. In a compound, atoms of different elements are chemically linked through strong covalent or ionic bonds, forming a new substance with unique properties. The properties of water, for example, are vastly different from those of hydrogen and oxygen gases.
In air, however, the gases are simply mixed together. They retain their individual properties and can be separated using physical methods. For instance, liquefaction of air involves cooling air to extremely low temperatures, causing its components to condense into liquids at different points. This process demonstrates that air is a mixture and not a compound. The components are not chemically bonded, they're simply present together.
The Significance of Air's Composition
The precise proportions of gases in the air are critical for maintaining life on Earth. The relatively high concentration of nitrogen prevents rapid combustion and supports a stable atmosphere. Oxygen's presence sustains aerobic respiration, providing energy for living organisms. Carbon dioxide's role in photosynthesis and the greenhouse effect are essential for regulating the planet's temperature and supporting plant life. Even the trace gases have significant influences, though often subtle, on various environmental processes.
Changes in the composition of air, especially increases in greenhouse gases like CO₂ and methane, have significant consequences for the global climate and the environment. These changes, primarily due to human activities, are leading to global warming, altered weather patterns, and other environmental challenges. Understanding the composition of air and the interactions between its components is therefore crucial for addressing these environmental concerns.
Exploring Air's Properties: A Mixture's Characteristics
The properties of air are a reflection of the properties of its constituent gases. Since air is a mixture, its properties are not fixed but vary depending on factors like altitude, location, and weather conditions. Some key properties include:
- Density: The density of air is relatively low compared to liquids and solids. This density varies with temperature and pressure.
- Compressibility: Air is readily compressible, meaning its volume can be reduced by applying pressure. This property is exploited in various applications, including pneumatic systems and scuba diving equipment.
- Expansibility: Air expands when heated and contracts when cooled. This property is fundamental to weather patterns and atmospheric circulation.
- Transmission of Sound: Air transmits sound waves, although the speed of sound varies with temperature and pressure.
- Solubility: Air's components have varying degrees of solubility in water, which affects the composition of aquatic environments.
Air Pollution: A Threat to Air Quality
Air pollution represents a significant alteration of air's natural composition. It introduces harmful pollutants, including particulate matter, ozone, sulfur dioxide, nitrogen oxides, and volatile organic compounds. These pollutants are released from various sources, including industrial emissions, vehicle exhaust, and natural processes like volcanic eruptions.
Air pollution poses serious threats to human health, causing respiratory problems, cardiovascular diseases, and other health issues. It also negatively impacts the environment, contributing to acid rain, ozone depletion, and climate change. Monitoring and controlling air pollution are therefore vital for protecting human health and the environment.
Conclusion: Air – A Dynamic and Essential Mixture
In conclusion, air is definitively a mixture, not a compound. The gases that make up air are not chemically bonded; instead, they exist as individual molecules, mixed together in a relatively constant proportion. This mixture, far from being inert, is a dynamic and complex system crucial for life on Earth. Its composition, constantly influenced by natural processes and human activities, deserves ongoing study and careful monitoring to ensure the preservation of our planet's atmosphere and the well-being of all living organisms. The delicate balance of gases within this mixture underscores the importance of environmental stewardship and the need for sustainable practices to maintain the health and quality of the air we breathe. Understanding the difference between elements, compounds, and mixtures, and recognizing air as a vital mixture, is critical for appreciating its significance and the responsibilities we have toward protecting it.
Latest Posts
Latest Posts
-
Least Common Multiple Of 21 And 28
Mar 14, 2025
-
How Many Valence Shell Electrons Does The Element Carbon Have
Mar 14, 2025
-
How Many Bones A Shark Have
Mar 14, 2025
-
In Situ Conservation Vs Ex Situ Conservation
Mar 14, 2025
-
What Is Enforcement Directorate In India
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Is Air An Element Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
