In The Burning Of Methane What Are The Reactants
Juapaving
Mar 28, 2025 · 5 min read
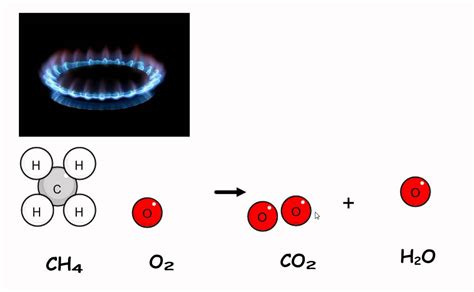
Table of Contents
In the Burning of Methane: What are the Reactants? A Deep Dive into Combustion Chemistry
Methane (CH₄), the simplest hydrocarbon, plays a crucial role in various aspects of our lives, from a potent greenhouse gas to a significant energy source. Understanding its combustion, specifically identifying the reactants and products, is fundamental to comprehending its environmental impact and its use in energy production. This detailed exploration delves into the chemical processes involved in methane burning, highlighting the essential reactants and the intricacies of the reaction.
Understanding Combustion: A Fundamental Chemical Process
Combustion, often referred to as burning, is a rapid chemical reaction between a substance (the fuel) and an oxidant (usually oxygen), releasing energy in the form of heat and light. This exothermic reaction involves the oxidation of the fuel, breaking its chemical bonds and forming new ones with oxygen. The products of complete combustion typically include carbon dioxide (CO₂), water (H₂O), and energy. Incomplete combustion, however, can yield other products like carbon monoxide (CO) and soot (carbon particles).
Methane Combustion: The Reactants
In the context of methane combustion, the reactants are straightforward:
-
Methane (CH₄): This is our fuel, a colorless, odorless gas composed of one carbon atom bonded to four hydrogen atoms. Methane is the primary component of natural gas and is found in various natural sources, including wetlands, landfills, and natural gas deposits. Its abundance makes it a significant energy source and a key player in the global carbon cycle.
-
Oxygen (O₂): This is the oxidant, essential for the combustion process. Oxygen is a diatomic molecule, meaning it exists as two oxygen atoms bonded together. Atmospheric air provides the oxygen required for most combustion reactions. The concentration of oxygen significantly impacts the completeness of combustion; sufficient oxygen leads to complete combustion, while a deficiency leads to incomplete combustion.
The Chemical Equation: A Simplified Representation
The balanced chemical equation for the complete combustion of methane is:
CH₄ + 2O₂ → CO₂ + 2H₂O + Heat
This equation represents the stoichiometry of the reaction, indicating the molar ratio of reactants to products. One mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide, two moles of water, and a significant amount of heat.
The Mechanism of Methane Combustion: A Complex Reaction
While the overall chemical equation provides a simplified representation, the actual combustion process is far more complex. It involves a series of elementary reactions, including chain initiation, chain propagation, and chain termination steps, forming various intermediate species.
Chain Initiation: The Starting Point
The combustion process begins with the initiation step. This usually involves the homolytic cleavage of a bond in a reactant molecule, creating free radicals. In methane combustion, this could involve the high-temperature dissociation of oxygen molecules into oxygen radicals:
O₂ → 2O•
Or the reaction of oxygen with methane:
CH₄ + O₂ → CH₃• + HO₂•
These highly reactive free radicals (indicated by the •) are crucial for initiating the subsequent chain propagation steps.
Chain Propagation: Building the Reaction
Chain propagation steps involve the reaction of free radicals with other reactant molecules, producing new free radicals that continue the chain reaction. These steps are crucial in the combustion process, leading to a rapid consumption of reactants and the production of products. Several propagation steps can occur, including:
CH₃• + O₂ → CH₃O₂•
CH₃O₂• + CH₄ → CH₃OOH + CH₃•
CH₃OOH → CH₃O• + •OH
•OH + CH₄ → CH₃• + H₂O
These steps demonstrate the formation of various intermediate species, such as methylperoxy radical (CH₃O₂•), methyl hydroperoxide (CH₃OOH), methoxy radical (CH₃O•), and hydroxyl radical (•OH). These reactive species then participate in further reactions, propagating the combustion chain.
Chain Termination: Bringing the Reaction to a Halt
Chain termination steps involve the combination of two free radicals, resulting in the formation of stable molecules and the cessation of the chain reaction. Examples of termination reactions include:
2•OH → H₂O₂
CH₃• + •OH → CH₃OH
2CH₃• → C₂H₆
These termination reactions decrease the concentration of free radicals, eventually slowing down and stopping the combustion process.
Factors Affecting Methane Combustion
Several factors influence the efficiency and completeness of methane combustion:
-
Temperature: Higher temperatures accelerate the reaction rate, promoting complete combustion.
-
Oxygen Concentration: Sufficient oxygen is vital for complete combustion. Oxygen deficiency can lead to the formation of incomplete combustion products like carbon monoxide (CO) and soot.
-
Pressure: Higher pressure increases the collision frequency between reactant molecules, potentially increasing the reaction rate.
-
Presence of Catalysts: Catalysts can lower the activation energy of the reaction, facilitating faster and more efficient combustion.
-
Mixing of Reactants: Effective mixing of methane and oxygen is crucial to ensure that all the fuel is completely combusted.
Incomplete Combustion: A Concern
Incomplete combustion occurs when there is insufficient oxygen for the complete oxidation of methane. This leads to the formation of carbon monoxide (CO) and/or elemental carbon (soot). Carbon monoxide is a highly toxic gas, while soot contributes to air pollution and respiratory problems. The chemical equations for incomplete combustion can vary greatly depending on the oxygen availability. Examples include:
2CH₄ + 3O₂ → 2CO + 4H₂O
CH₄ + O₂ → C + 2H₂O
These equations show that incomplete combustion results in less energy release compared to complete combustion and generates harmful byproducts.
Environmental Implications of Methane Combustion
The combustion of methane releases carbon dioxide (CO₂), a major greenhouse gas that contributes to climate change. Although methane itself is a more potent greenhouse gas than CO₂, its shorter atmospheric lifetime means that the overall warming effect of methane combustion is heavily influenced by the amount of CO₂ released. Furthermore, incomplete combustion adds the environmental burden of carbon monoxide and soot pollution.
Conclusion: A Comprehensive Look at Methane Burning
The burning of methane, while seemingly simple from its overall chemical equation, is a complex chemical process involving multiple elementary reactions and influenced by various factors. Understanding the reactants, the reaction mechanism, and the environmental consequences of both complete and incomplete combustion is crucial for managing the use of methane as an energy source and mitigating its impact on the environment. Further research and technological advancements are constantly being pursued to improve the efficiency and minimize the negative environmental effects of methane combustion. This ongoing effort reflects the critical importance of comprehending this fundamental chemical reaction in the context of our energy needs and environmental stewardship.
Latest Posts
Latest Posts
-
What Medical Problem Afflicts Mrs Mallard
Mar 31, 2025
-
2 Over 5 As A Percent
Mar 31, 2025
-
35 Inches Is How Many Feet
Mar 31, 2025
-
3 Cm Is How Many Mm
Mar 31, 2025
-
How Many Lines Of Symmetry Are In A Star
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about In The Burning Of Methane What Are The Reactants . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
