In Mechanism Photophosphorylation Is Most Similar To
Juapaving
Mar 06, 2025 · 6 min read
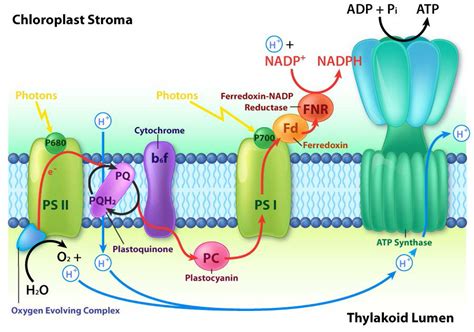
Table of Contents
In What Mechanism is Photophosphorylation Most Similar To? A Deep Dive into Chemiosmosis
Photophosphorylation, the process by which light energy is converted into the chemical energy of ATP in photosynthetic organisms, is a fascinating and crucial aspect of life on Earth. Understanding its mechanism reveals a striking similarity to another fundamental biological process: oxidative phosphorylation. While occurring in different cellular compartments and utilizing different electron sources, both processes share the core principle of chemiosmosis, a remarkable feat of cellular engineering that harnesses the power of proton gradients to synthesize ATP.
This article will delve into the intricate details of photophosphorylation, highlighting its similarities and subtle differences with oxidative phosphorylation, explaining the underlying mechanism of chemiosmosis, and exploring the crucial role of various components involved in both processes.
The Core Principle: Chemiosmosis – The Proton Motive Force
At the heart of both photophosphorylation and oxidative phosphorylation lies chemiosmosis. This ingenious mechanism utilizes a proton gradient (a difference in proton concentration) across a membrane to drive ATP synthesis. The protons (H⁺ ions) are actively pumped across a membrane, creating an electrochemical gradient. This gradient consists of two components:
- Chemical Gradient: A difference in proton concentration across the membrane. The side with higher proton concentration has a lower pH.
- Electrical Gradient: A difference in charge across the membrane, created by the movement of positively charged protons.
This combined gradient is termed the proton motive force (PMF). It represents stored potential energy that can be harnessed to perform work. In both photophosphorylation and oxidative phosphorylation, this work is the synthesis of ATP.
Photophosphorylation: Light-Driven ATP Synthesis
Photophosphorylation occurs in the thylakoid membranes within chloroplasts of photosynthetic organisms. It's divided into two phases:
1. Non-cyclic Photophosphorylation: The Z-Scheme
This pathway is the primary route for ATP and NADPH production. It involves two photosystems, Photosystem II (PSII) and Photosystem I (PSI), working in series:
-
Photosystem II (PSII): Light energy excites chlorophyll molecules in PSII, causing electrons to be released. These electrons are passed along an electron transport chain (ETC). The electron loss from PSII is replenished by splitting water molecules (photolysis), releasing oxygen as a byproduct. This water-splitting process also releases protons (H⁺) into the thylakoid lumen, contributing to the proton gradient.
-
Electron Transport Chain (ETC): As electrons move down the ETC, energy is released and used to pump protons from the stroma into the thylakoid lumen, further increasing the proton gradient. Electron carriers like plastoquinone (PQ) and cytochrome b6f complex are key components of this chain.
-
Photosystem I (PSI): The electrons from the ETC reach PSI, where they are re-excited by light energy. These high-energy electrons are then passed to ferredoxin (Fd) and subsequently used to reduce NADP⁺ to NADPH, a crucial reducing agent in the Calvin cycle.
-
ATP Synthase: The proton gradient established across the thylakoid membrane drives protons back into the stroma through ATP synthase, an enzyme that uses the energy of the proton flow to synthesize ATP from ADP and inorganic phosphate (Pi). This process is analogous to the rotary engine, with the movement of protons causing a conformational change in ATP synthase, leading to ATP production.
2. Cyclic Photophosphorylation: ATP Production without NADPH
Under certain conditions, particularly when the demand for ATP is high and NADPH is less needed, cyclic photophosphorylation occurs. Here, the electrons from PSI are not passed to NADP⁺ but instead are cycled back to the ETC, contributing to further proton pumping and ATP synthesis. This pathway generates additional ATP without producing NADPH.
Oxidative Phosphorylation: Respiration-Driven ATP Synthesis
Oxidative phosphorylation takes place in the inner mitochondrial membrane. It's the final stage of cellular respiration, where the energy stored in NADH and FADH2 (generated during glycolysis and the Krebs cycle) is used to produce ATP:
-
Electron Transport Chain (ETC): Electrons from NADH and FADH2 enter the ETC at different points. As they move down the chain, energy is released and used to pump protons from the mitochondrial matrix into the intermembrane space, building a proton gradient. Cytochromes and other electron carriers facilitate this electron transport.
-
ATP Synthase: The proton gradient across the inner mitochondrial membrane drives protons back into the matrix through ATP synthase, leading to ATP synthesis. This process is essentially the same as in photophosphorylation – utilizing the PMF to drive ATP synthesis.
-
Oxygen as the Final Electron Acceptor: In oxidative phosphorylation, oxygen serves as the final electron acceptor at the end of the ETC. It combines with electrons and protons to form water.
Similarities Between Photophosphorylation and Oxidative Phosphorylation:
- Chemiosmosis: Both processes rely on chemiosmosis to synthesize ATP. A proton gradient drives ATP synthesis through ATP synthase.
- Electron Transport Chain: Both processes involve an electron transport chain where electrons are passed along a series of carriers, releasing energy used for proton pumping.
- ATP Synthase: Both processes utilize ATP synthase, a remarkable molecular machine, to convert the energy stored in the proton gradient into ATP.
- Proton Gradient Generation: Both processes create a proton gradient across a membrane, building up a PMF.
- Energy Conversion: Both convert energy from one form into another. Photophosphorylation converts light energy into chemical energy (ATP), while oxidative phosphorylation converts chemical energy (from NADH and FADH2) into chemical energy (ATP).
Differences Between Photophosphorylation and Oxidative Phosphorylation:
- Electron Source: Photophosphorylation utilizes light energy to excite electrons from water, while oxidative phosphorylation uses electrons from NADH and FADH2, derived from the breakdown of organic molecules.
- Location: Photophosphorylation occurs in the thylakoid membrane of chloroplasts, while oxidative phosphorylation occurs in the inner mitochondrial membrane.
- Final Electron Acceptor: The final electron acceptor in photophosphorylation is NADP⁺, while in oxidative phosphorylation, it is oxygen.
- Byproducts: Photophosphorylation produces oxygen as a byproduct, whereas oxidative phosphorylation produces water.
- Light Dependency: Photophosphorylation is directly dependent on light, while oxidative phosphorylation is not.
The Crucial Role of ATP Synthase: A Molecular Marvel
ATP synthase is a remarkable enzyme complex common to both photophosphorylation and oxidative phosphorylation. Its structure and function are remarkably conserved across different organisms. It comprises two main components:
- F₀ unit: Embedded in the membrane, this unit forms a channel for proton flow.
- F₁ unit: Protrudes into the stroma (chloroplasts) or matrix (mitochondria), containing the catalytic sites for ATP synthesis.
The flow of protons through the F₀ unit causes a rotation of a central stalk, inducing conformational changes in the F₁ unit, leading to the synthesis of ATP from ADP and Pi. This rotational catalysis is a beautiful example of the elegance and efficiency of biological machinery.
Conclusion: A Tale of Two Processes
Photophosphorylation and oxidative phosphorylation, while distinct in their specific components and locations, share a remarkable commonality: the utilization of chemiosmosis to synthesize ATP. This underlying mechanism highlights the elegance and efficiency of biological systems in harnessing energy gradients to power life's processes. Understanding these similarities and differences provides crucial insights into the fundamental mechanisms of energy conversion in living organisms, underlining the interconnectedness of photosynthesis and respiration in shaping the biosphere. Further research continues to unravel the intricate details of these processes, revealing ever more intricate layers of biological complexity and ingenuity.
Latest Posts
Latest Posts
-
What Is The Sum Of Interior Angles Of A Hexagon
Mar 06, 2025
-
Shape With A Square Base And Four Triangular Faces
Mar 06, 2025
-
Words That Have Oi In It
Mar 06, 2025
-
Hydrogen Is Metal Nonmetal Or Metalloids
Mar 06, 2025
-
The Correct Sequence Of Events In Viral Multiplication Is
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about In Mechanism Photophosphorylation Is Most Similar To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
