In A Solution The Solvent Is
Juapaving
Mar 21, 2025 · 6 min read
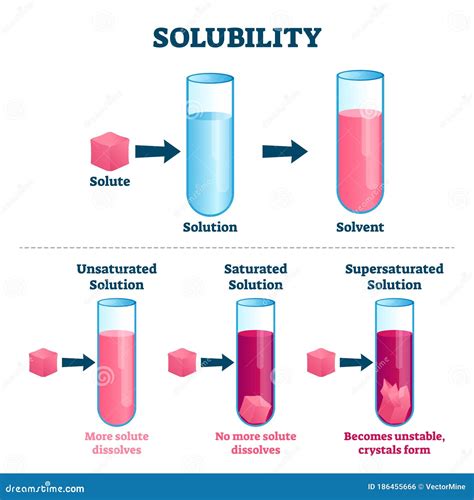
Table of Contents
In a Solution, the Solvent Is… Understanding the Fundamentals of Solutions
Solutions are ubiquitous in chemistry and everyday life. From the air we breathe to the blood in our veins, solutions are mixtures where one substance dissolves into another. Understanding the components of a solution, particularly the role of the solvent, is crucial for grasping numerous chemical and physical processes. This comprehensive guide dives deep into the nature of solvents and their significance in solution chemistry.
What is a Solution? A Deep Dive into Mixtures
A solution is a homogeneous mixture composed of two or more substances. This means the components are uniformly dispersed at a molecular level, resulting in a single phase (e.g., liquid, gas, or solid). Crucially, the components of a solution cannot be easily separated by physical methods like filtration. Instead, techniques like distillation or chromatography are usually required.
To fully grasp the concept of a solution, we need to understand its key components:
- Solute: This is the substance that dissolves in the solvent. It's usually present in a smaller amount compared to the solvent. Examples include salt (NaCl) in saltwater, sugar in sweet tea, or oxygen (O2) in air.
- Solvent: This is the substance that dissolves the solute. It's typically present in a larger amount and determines the physical state of the solution. Water is the most common solvent, but others exist for various applications.
The Importance of the Solvent
The solvent plays a critical role in solution formation. Its properties dictate:
- Solubility: The ability of a solute to dissolve in a solvent. Polar solvents like water tend to dissolve polar solutes (e.g., salts, sugars), while nonpolar solvents like hexane dissolve nonpolar solutes (e.g., oils, fats). This is governed by the principle of "like dissolves like."
- Solution Concentration: The amount of solute dissolved in a given amount of solvent. Concentration is expressed in various units, such as molarity (moles of solute per liter of solution), molality (moles of solute per kilogram of solvent), and percent by mass.
- Solution Properties: The physical and chemical properties of a solution, such as its density, boiling point, freezing point, and conductivity, are influenced significantly by the solvent's properties and the concentration of the solute.
Types of Solvents: Exploring the Diverse World of Solvents
Solvents come in a vast array of types, categorized by their chemical properties and applications:
1. Polar Solvents: The Water World and Beyond
Polar solvents possess a significant dipole moment, meaning they have a partial positive and a partial negative charge within the molecule. This polarity allows them to effectively dissolve polar solutes through strong dipole-dipole interactions and hydrogen bonding.
- Water (H₂O): The quintessential polar solvent, indispensable in biological systems and numerous industrial processes. Its exceptional ability to dissolve a wide range of substances is due to its strong polarity and hydrogen bonding capabilities.
- Ethanol (CH₃CH₂OH): A common polar solvent used in beverages, pharmaceuticals, and as a cleaning agent. It's miscible with water, meaning they mix completely in any proportion.
- Acetone (CH₃COCH₃): A highly versatile polar solvent used in various industries, including paint thinners, nail polish remover, and chemical synthesis.
2. Nonpolar Solvents: The Realm of Hydrophobic Interactions
Nonpolar solvents have little to no dipole moment, meaning their electron distribution is relatively even. They effectively dissolve nonpolar solutes through weak London dispersion forces.
- Hexane (C₆H₁₄): A common nonpolar solvent used in the extraction of oils and fats.
- Benzene (C₆H₆): A historically important nonpolar solvent, although its use is declining due to its carcinogenic properties.
- Toluene (C₇H₈): A nonpolar solvent used as a paint thinner and in various industrial processes.
3. Protic vs. Aprotic Solvents: A Matter of Hydrogen Bonding
Solvents are also categorized as either protic or aprotic, depending on their ability to donate a proton (H⁺).
- Protic solvents have an acidic hydrogen atom directly bonded to an electronegative atom (like oxygen or nitrogen). These solvents readily participate in hydrogen bonding. Examples include water, ethanol, and methanol.
- Aprotic solvents lack an acidic hydrogen atom and do not participate in hydrogen bonding as effectively. Examples include acetone, dimethyl sulfoxide (DMSO), and acetonitrile.
The choice of protic versus aprotic solvent is crucial in various chemical reactions, as the solvent's ability to donate or accept protons can significantly influence reaction rates and selectivity.
Solvent Selection: Choosing the Right Solvent for the Job
Selecting the appropriate solvent for a particular application is a critical step in many chemical and physical processes. Several factors need to be considered:
- Solubility of the Solute: The solvent must effectively dissolve the target solute. This often hinges on the principle of "like dissolves like."
- Solvent Properties: The solvent's boiling point, viscosity, density, and other physical properties influence the ease of handling and separation.
- Chemical Compatibility: The solvent must be chemically inert with respect to the solute and any other components in the system.
- Toxicity and Environmental Impact: The solvent's toxicity and environmental impact must be considered, with safer alternatives preferred whenever possible.
- Cost and Availability: The cost and availability of the solvent must be evaluated, particularly in large-scale applications.
The Solvent's Role in Chemical Reactions
The solvent plays a far more significant role than merely dissolving the reactants. It often actively participates in the reaction mechanism, influencing:
- Reaction Rate: The solvent can stabilize or destabilize transition states, impacting the reaction rate. Protic solvents, for example, can accelerate reactions involving nucleophiles by stabilizing the transition state.
- Reaction Selectivity: The solvent can influence the formation of different products by preferentially stabilizing certain intermediates or transition states.
- Reaction Mechanism: The solvent can participate directly in the reaction mechanism, acting as a reactant or a catalyst.
Examples of Solutions in Everyday Life
Solutions are omnipresent in everyday life. Here are some common examples:
- Saltwater: Salt (NaCl) is the solute, and water (H₂O) is the solvent.
- Sweet Tea: Sugar is the solute, and water is the solvent.
- Air: Various gases, like oxygen and nitrogen, are dissolved in each other.
- Blood: Various substances, including proteins, glucose, and electrolytes, are dissolved in water.
- Cleaning Solutions: Many cleaning agents are solutions, with various surfactants and other components dissolved in water.
Conclusion: The Unsung Hero of Chemistry
In a solution, the solvent is the unsung hero, its properties dictating solubility, concentration, and the overall behavior of the system. Understanding the different types of solvents and their properties is crucial for anyone working in chemistry, biology, or related fields. From selecting the appropriate solvent for a particular application to understanding its role in complex chemical reactions, the solvent's influence is profound and far-reaching. Mastering the concepts surrounding solutions and the pivotal role of the solvent is essential for a deeper appreciation of the chemical world around us.
Latest Posts
Latest Posts
-
Which Of The Following Is An Abstract Word
Mar 27, 2025
-
What Type Of Wave Is Water Wave
Mar 27, 2025
-
How Many Electrons Are Shared By A Triple Bond
Mar 27, 2025
-
What Is The Normal Balance Of Accumulated Depreciation
Mar 27, 2025
-
How Many Feet Is In 30 Yards
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about In A Solution The Solvent Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
