How Many Electrons Are Shared By A Triple Bond
Juapaving
Mar 27, 2025 · 6 min read
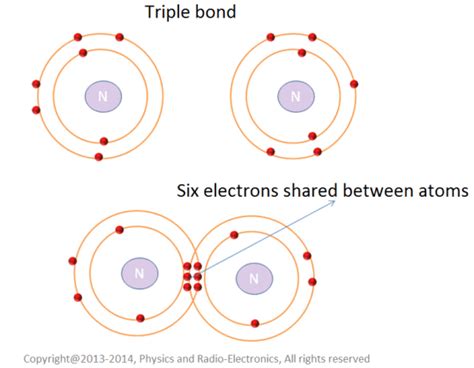
Table of Contents
How Many Electrons Are Shared by a Triple Bond? Delving into Covalent Bonding
Understanding chemical bonding is fundamental to grasping the behavior of matter. Among the various types of chemical bonds, covalent bonds, where atoms share electrons to achieve a stable electron configuration, are particularly prevalent. A key aspect of covalent bonding involves the concept of multiple bonds, specifically triple bonds. This article dives deep into the intricacies of triple bonds, exploring how many electrons are shared and the implications of this sharing for molecular structure and properties.
What is a Covalent Bond?
Before we delve into triple bonds, let's establish a solid foundation by defining a covalent bond. A covalent bond is a chemical bond formed by the sharing of one or more pairs of electrons between two atoms. This sharing allows both atoms to achieve a more stable electron configuration, typically resembling the stable electron configuration of a noble gas (a full outer electron shell). The strength of a covalent bond depends on the number of electron pairs shared and the electronegativity difference between the atoms involved.
Types of Covalent Bonds: Single, Double, and Triple Bonds
Covalent bonds are not limited to the sharing of a single electron pair. Instead, atoms can share multiple pairs of electrons, leading to different types of covalent bonds:
- Single Bond: Involves the sharing of one pair of electrons (two electrons). Represented by a single line (-) between the atoms.
- Double Bond: Involves the sharing of two pairs of electrons (four electrons). Represented by two lines (=) between the atoms.
- Triple Bond: Involves the sharing of three pairs of electrons (six electrons). Represented by three lines (≡) between the atoms.
The Essence of a Triple Bond: Six Shared Electrons
The core answer to the question "How many electrons are shared by a triple bond?" is six. A triple bond is characterized by the sharing of three pairs of electrons between two atoms. This significant electron sharing results in a very strong bond, influencing the molecule's properties in several ways.
Understanding the Formation of a Triple Bond
The formation of a triple bond occurs when each participating atom contributes three electrons to the shared pool. This allows each atom to effectively fill its valence shell (outermost electron shell), resulting in greater stability. Let's consider the classic example of nitrogen gas (N₂).
Nitrogen Gas (N₂) as a Prime Example
Nitrogen atoms have five electrons in their outermost shell. To achieve a stable octet (eight electrons in the outer shell), each nitrogen atom needs to gain three more electrons. This is achieved by sharing three pairs of electrons with another nitrogen atom, forming a triple bond (N≡N). Each nitrogen atom contributes three electrons to the bond, resulting in a total of six shared electrons.
This triple bond is exceptionally strong, explaining nitrogen gas's inertness and its abundance in the Earth's atmosphere. The strong bond requires significant energy to break, contributing to nitrogen's relatively low reactivity at standard temperature and pressure.
Implications of Triple Bonds on Molecular Properties
The presence of a triple bond significantly impacts the physical and chemical properties of a molecule. Here are some key implications:
-
Bond Strength: Triple bonds are stronger than double bonds, which are in turn stronger than single bonds. This increased bond strength stems from the greater number of shared electrons and the increased electron density between the bonded atoms. The stronger the bond, the more energy is needed to break it.
-
Bond Length: Triple bonds are shorter than double bonds, which are shorter than single bonds. The increased electron density between the atoms in a triple bond pulls the atoms closer together.
-
Reactivity: Molecules with triple bonds tend to be more reactive than those with single or double bonds, particularly in addition reactions. The high electron density and the relative instability of the triple bond make it susceptible to reactions where electrons are added.
-
Molecular Geometry: The presence of a triple bond significantly influences the molecular geometry. Triple bonds are linear, meaning the atoms involved in the triple bond lie in a straight line. This linearity affects the overall shape and polarity of the molecule.
-
Spectral Properties: Triple bonds exhibit characteristic absorption bands in infrared (IR) and Raman spectroscopy, which can be used for their identification and analysis. These spectral features arise from the vibrational modes of the triple bond.
Beyond Nitrogen: Other Molecules with Triple Bonds
While nitrogen gas is a quintessential example of a molecule with a triple bond, many other molecules also exhibit this bonding arrangement. Here are a few examples:
-
Alkynes: These hydrocarbons contain at least one carbon-carbon triple bond (C≡C). The simplest alkyne is ethyne (acetylene), with the formula C₂H₂.
-
Cyanides: These compounds contain a carbon-nitrogen triple bond (C≡N). Hydrogen cyanide (HCN) is a simple and highly toxic example.
-
Nitriles: Similar to cyanides, nitriles also feature a carbon-nitrogen triple bond. However, the nitrogen atom is attached to a carbon atom within a larger organic structure.
-
Carbon Monoxide (CO): This molecule exhibits a carbon-oxygen triple bond (C≡O). This strong bond contributes to the toxicity of carbon monoxide.
Further Exploration: Advanced Concepts
The understanding of triple bonds extends beyond the simple counting of electrons. Deeper explorations involve considering:
-
Orbital Hybridization: The atomic orbitals involved in the formation of a triple bond undergo hybridization, resulting in a specific arrangement of orbitals that optimize the overlap and sharing of electrons. For example, in the case of acetylene, the carbon atoms utilize sp hybridization.
-
Bond Order: This represents the number of bonds between two atoms, reflecting the overall strength of the bond. A triple bond has a bond order of 3, indicating a strong interaction.
-
Molecular Orbital Theory: This advanced model provides a more nuanced understanding of the electronic structure of molecules, including the distribution of electrons within bonding and anti-bonding orbitals.
-
Resonance Structures: In some cases, molecules may exhibit resonance, where the actual electronic structure is a hybrid of several possible Lewis structures, influencing the overall bond order and properties.
Conclusion: A Powerful Bond with Wide-Ranging Effects
A triple bond, with its six shared electrons, represents a strong and significant type of covalent bond. It profoundly impacts the physical and chemical properties of molecules, influencing bond strength, bond length, reactivity, molecular geometry, and spectral characteristics. Understanding the intricacies of triple bonds is critical for comprehending the behavior of a wide array of molecules, from simple diatomic gases to complex organic compounds. This fundamental knowledge forms a cornerstone of chemistry and has significant implications in various scientific fields, including materials science, organic chemistry, and biochemistry. Further exploration into the advanced concepts mentioned above can provide an even deeper and more complete understanding of this crucial chemical bond.
Latest Posts
Latest Posts
-
Greatest Common Factor Of 24 And 30
Mar 30, 2025
-
What Are All Of The Factors Of 49
Mar 30, 2025
-
Lowest Common Factor Of 3 And 8
Mar 30, 2025
-
Five Letter Words Starting With Ra
Mar 30, 2025
-
What Is The Chemical Formula For Phosphorus Pentachloride
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are Shared By A Triple Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
