How To Convert Pka To Ph
Juapaving
Mar 22, 2025 · 5 min read
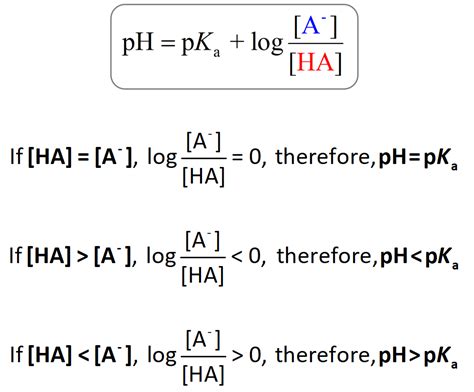
Table of Contents
- How To Convert Pka To Ph
- Table of Contents
- How to Convert pKa to pH: A Comprehensive Guide
- Understanding pKa and pH: The Fundamentals
- What is pKa?
- What is pH?
- The Relationship Between pKa and pH: The Henderson-Hasselbalch Equation
- Converting pKa to pH: A Step-by-Step Approach
- Special Cases and Considerations
- 1. Strong Acids and Bases:
- 2. Buffer Solutions:
- 3. Dilute Solutions:
- 4. Temperature Dependence:
- Beyond the Basics: More Complex Scenarios
- Applications of pKa and pH Conversions
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
How to Convert pKa to pH: A Comprehensive Guide
Understanding the relationship between pKa and pH is crucial in various fields, including chemistry, biochemistry, and environmental science. While they might seem interchangeable at first glance, they represent distinct yet interconnected concepts. This comprehensive guide will delve into the intricacies of pKa and pH, explaining their definitions, the relationship between them, and ultimately, how to convert pKa to pH under different circumstances.
Understanding pKa and pH: The Fundamentals
Before we tackle the conversion process, let's solidify our understanding of these two fundamental concepts:
What is pKa?
pKa is a quantitative measure of the acidity of a given acid. It represents the negative logarithm (base 10) of the acid dissociation constant (Ka). The Ka value reflects the equilibrium between the undissociated acid (HA) and its conjugate base (A⁻) and hydrogen ions (H⁺) in an aqueous solution:
HA ⇌ H⁺ + A⁻
A lower pKa value indicates a stronger acid, meaning it readily donates protons (H⁺). Conversely, a higher pKa value signifies a weaker acid, which holds onto its protons more tightly. The pKa value is specific to a particular acid at a given temperature.
What is pH?
pH, on the other hand, measures the acidity or alkalinity of a solution. It's also a negative logarithm (base 10) but of the hydrogen ion concentration ([H⁺]) in the solution:
pH = -log₁₀[H⁺]
A lower pH indicates a more acidic solution, with a higher concentration of H⁺ ions. A higher pH represents a more alkaline (basic) solution, with a lower concentration of H⁺ ions. A pH of 7 is considered neutral at 25°C.
The Relationship Between pKa and pH: The Henderson-Hasselbalch Equation
The key to understanding the relationship and ultimately converting pKa to pH lies in the Henderson-Hasselbalch equation:
pH = pKa + log₁₀([A⁻]/[HA])
This equation beautifully links the pH of a solution containing a weak acid (HA) and its conjugate base (A⁻) to its pKa. Let's break down the components:
- pH: The pH of the solution. This is what we aim to calculate.
- pKa: The acid dissociation constant of the weak acid. This is a known value for a specific acid.
- [A⁻]: The concentration of the conjugate base.
- [HA]: The concentration of the undissociated acid.
Converting pKa to pH: A Step-by-Step Approach
The direct conversion of pKa to pH isn't straightforward because it depends on the ratio of the conjugate base to the acid ([A⁻]/[HA]). Therefore, to calculate pH from pKa, we need additional information about the concentration of the acid and its conjugate base.
Let's illustrate this with a step-by-step example:
Example: Calculate the pH of a solution containing 0.1 M acetic acid (CH₃COOH) and 0.2 M sodium acetate (CH₃COONa). The pKa of acetic acid is 4.76.
Steps:
-
Identify the components: We have a weak acid (acetic acid, CH₃COOH) and its conjugate base (acetate ion, CH₃COO⁻ from sodium acetate).
-
Determine the concentrations: [HA] = [CH₃COOH] = 0.1 M and [A⁻] = [CH₃COO⁻] = 0.2 M.
-
Apply the Henderson-Hasselbalch equation:
pH = pKa + log₁₀([A⁻]/[HA]) pH = 4.76 + log₁₀(0.2 M / 0.1 M) pH = 4.76 + log₁₀(2) pH = 4.76 + 0.30 pH = 5.06
Therefore, the pH of this solution is approximately 5.06.
Special Cases and Considerations
The Henderson-Hasselbalch equation works best for weak acids and their conjugate bases in solutions where the concentration of the acid and its conjugate base are comparable. Several scenarios require special consideration:
1. Strong Acids and Bases:
The Henderson-Hasselbalch equation is not applicable to strong acids and bases because they completely dissociate in water. For strong acids, the pH is directly calculated from the concentration of H⁺ ions. For strong bases, the pOH is calculated first, then converted to pH.
2. Buffer Solutions:
Buffer solutions are designed to resist changes in pH upon the addition of small amounts of acid or base. These solutions typically consist of a weak acid and its conjugate base (or a weak base and its conjugate acid) in approximately equal concentrations. The Henderson-Hasselbalch equation is particularly useful in calculating the pH of buffer solutions.
3. Dilute Solutions:
When dealing with very dilute solutions, the autoionization of water can significantly affect the pH, especially when the pH approaches neutrality. In these cases, you might need to account for the contribution of water's autoionization to the overall [H⁺] concentration.
4. Temperature Dependence:
Remember that both pKa and pH are temperature-dependent. The pKa of an acid changes with temperature, which directly impacts the pH calculation. Always ensure you're using the pKa value appropriate for the temperature of your solution.
Beyond the Basics: More Complex Scenarios
The conversion of pKa to pH can become more complex when dealing with polyprotic acids (acids with more than one ionizable proton), mixtures of acids and bases, or solutions containing other ions that could affect the equilibrium. In such cases, more advanced techniques like numerical methods or simulation software might be necessary to obtain accurate pH calculations. These methods are beyond the scope of a basic guide but highlight the need for understanding the underlying chemical principles.
Applications of pKa and pH Conversions
Understanding the relationship between pKa and pH is critical in many applications:
- Drug Discovery and Development: The pKa of drugs significantly impacts their absorption, distribution, metabolism, and excretion (ADME) properties within the body.
- Environmental Monitoring: pH measurements are crucial in assessing the quality of water bodies and the impact of pollution. Knowing the pKa of various substances helps predict their behavior in these environments.
- Analytical Chemistry: Titration curves, used in quantitative analysis, rely heavily on the understanding of pKa and pH changes during acid-base reactions.
- Biochemistry: Many biochemical processes are pH-sensitive. Maintaining a specific pH range is critical for enzyme activity and overall biological function.
Conclusion
Converting pKa to pH requires a clear understanding of the Henderson-Hasselbalch equation and the underlying principles of acid-base chemistry. This guide provides a comprehensive overview of the conversion process, including special cases and considerations. While the basic calculation is straightforward, remember that the accuracy of the pH calculation hinges on the accuracy of the known pKa value, concentrations, and the consideration of any complicating factors in the system. With practice and a thorough understanding of the underlying concepts, you can confidently perform these conversions and apply them to a wide range of scientific and practical scenarios.
Latest Posts
Latest Posts
-
Sum Of Even And Odd Numbers
Mar 23, 2025
-
Least Common Multiple Of 21 And 24
Mar 23, 2025
-
Rank The Following Chemical Bonds According To Their Strength
Mar 23, 2025
-
Least Common Multiple Of 15 And 21
Mar 23, 2025
-
How To Convert Hexadecimal To Octal
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How To Convert Pka To Ph . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
