How To Calculate Moles Of An Element
Juapaving
Mar 08, 2025 · 5 min read
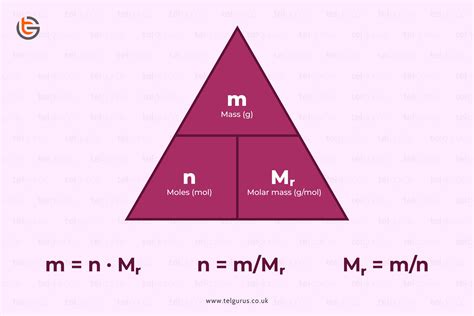
Table of Contents
How to Calculate Moles of an Element: A Comprehensive Guide
Understanding moles is fundamental to mastering chemistry. The mole (mol) is the International System of Units (SI) base unit for the amount of substance. It's not just a random number; it's a crucial concept that connects the microscopic world of atoms and molecules to the macroscopic world we observe in the lab. This comprehensive guide will walk you through various methods of calculating moles of an element, clarifying the intricacies and providing practical examples.
Understanding the Mole Concept
Before diving into calculations, let's solidify our understanding of what a mole represents. One mole of any substance contains Avogadro's number of particles – that's approximately 6.022 x 10²³ particles. These particles can be atoms, molecules, ions, or even formula units, depending on the substance in question. Think of it like a dozen (12) – a dozen eggs always contains 12 eggs, regardless of their size or type. Similarly, a mole of carbon atoms always contains 6.022 x 10²³ carbon atoms.
Key Formulas for Calculating Moles
The core formula for calculating moles is elegantly simple:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
Let's break down each component:
-
Mass (g): This is the mass of the substance you're working with, measured in grams (g). Accurate measurement is paramount for obtaining accurate results.
-
Molar Mass (g/mol): This is the mass of one mole of a substance. It's calculated by adding the atomic masses (found on the periodic table) of all the atoms in the chemical formula. For an element, the molar mass is simply its atomic mass expressed in grams per mole.
Calculating Moles Using Mass and Molar Mass
This is the most common method for calculating moles. Let's illustrate with some examples:
Example 1: Calculating Moles of Gold (Au)
Suppose you have 197 grams of gold (Au). The atomic mass of gold from the periodic table is approximately 197 g/mol.
-
Identify the mass: Mass = 197 g
-
Find the molar mass: Molar mass (Au) = 197 g/mol
-
Apply the formula: Moles = Mass / Molar Mass = 197 g / 197 g/mol = 1 mol
Therefore, 197 grams of gold contain 1 mole of gold atoms.
Example 2: Calculating Moles of Iron (Fe)
Let's say you have 55.85 grams of iron (Fe). The atomic mass of iron is approximately 55.85 g/mol.
-
Identify the mass: Mass = 55.85 g
-
Find the molar mass: Molar mass (Fe) = 55.85 g/mol
-
Apply the formula: Moles = Mass / Molar Mass = 55.85 g / 55.85 g/mol = 1 mol
Thus, 55.85 grams of iron contain 1 mole of iron atoms.
Example 3: Calculating Moles of a Larger Mass
Now, let's consider a larger mass. Suppose you have 1000 grams of aluminum (Al). The atomic mass of aluminum is approximately 27 g/mol.
-
Identify the mass: Mass = 1000 g
-
Find the molar mass: Molar mass (Al) = 27 g/mol
-
Apply the formula: Moles = Mass / Molar Mass = 1000 g / 27 g/mol ≈ 37.04 mol
Therefore, 1000 grams of aluminum contain approximately 37.04 moles of aluminum atoms.
Calculating Moles Using Number of Atoms
You can also calculate the number of moles if you know the number of atoms present. The formula for this is:
Moles (mol) = Number of Atoms / Avogadro's Number
Example 4: Calculating Moles from the Number of Atoms
Let's say you have 1.2044 x 10²⁴ atoms of copper (Cu).
-
Identify the number of atoms: Number of atoms = 1.2044 x 10²⁴ atoms
-
Use Avogadro's Number: Avogadro's Number = 6.022 x 10²³ atoms/mol
-
Apply the formula: Moles = Number of Atoms / Avogadro's Number = (1.2044 x 10²⁴ atoms) / (6.022 x 10²³ atoms/mol) = 2 mol
Therefore, 1.2044 x 10²⁴ atoms of copper represent 2 moles of copper.
Calculating Moles in More Complex Scenarios
While the above examples focus on single elements, the mole concept extends to compounds and mixtures as well. The molar mass of a compound is the sum of the atomic masses of all the atoms in its chemical formula.
Example 5: Moles in a Compound
Let's consider water (H₂O). To calculate the molar mass of water:
- Atomic mass of Hydrogen (H): 1 g/mol (approximately)
- Atomic mass of Oxygen (O): 16 g/mol (approximately)
Molar mass (H₂O) = (2 x 1 g/mol) + (1 x 16 g/mol) = 18 g/mol
If you have 36 grams of water:
Moles = Mass / Molar Mass = 36 g / 18 g/mol = 2 mol
Practical Applications of Mole Calculations
The ability to calculate moles is critical in many areas of chemistry and related fields:
-
Stoichiometry: Mole calculations are the foundation of stoichiometry, which allows us to determine the quantitative relationships between reactants and products in chemical reactions.
-
Titrations: In titrations, mole calculations are essential to determine the concentration of unknown solutions.
-
Gas Laws: The ideal gas law (PV = nRT) uses moles (n) to relate the pressure, volume, and temperature of gases.
-
Solution Concentration: Molarity (moles per liter) is a common way to express the concentration of solutions.
Avoiding Common Mistakes
-
Unit Consistency: Ensure all units are consistent (grams for mass, g/mol for molar mass).
-
Significant Figures: Pay attention to significant figures throughout your calculations. The final answer should reflect the precision of the input data.
-
Molar Mass Accuracy: Use accurate atomic masses from a reliable periodic table.
Conclusion
Calculating moles is a fundamental skill in chemistry. By mastering the concepts presented here – understanding Avogadro's number, using the core formula, and applying it to various scenarios – you'll build a strong foundation for tackling more advanced chemical calculations. Remember to practice regularly and always double-check your work to minimize errors. The ability to accurately calculate moles opens doors to a deeper understanding of chemical reactions and quantitative analysis. This skill is invaluable not only for academic pursuits but also for numerous applications in various scientific and industrial fields.
Latest Posts
Latest Posts
-
What Is The Prime Factorization Of 180
Mar 09, 2025
-
How Many Sides Are In A Quadrilateral
Mar 09, 2025
-
How Are Molecules Different From Compounds
Mar 09, 2025
-
What Is The Factor Of 31
Mar 09, 2025
-
In What Hemisphere Is Australia Located
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Moles Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
