How Many Valence Electrons In Sr
Juapaving
Mar 31, 2025 · 6 min read
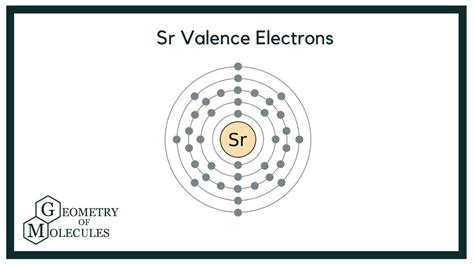
Table of Contents
How Many Valence Electrons Does Strontium (Sr) Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Strontium (Sr), an alkaline earth metal, plays a fascinating role in various applications, from fireworks to nuclear medicine. Understanding its chemical behavior hinges on knowing its valence electron configuration. This article delves deep into the electronic structure of strontium, explaining its valence electrons, their significance in chemical bonding, and how this influences strontium's properties and applications.
Understanding Valence Electrons
Before focusing specifically on strontium, let's establish a foundational understanding of valence electrons. Valence electrons are the outermost electrons in an atom. These electrons are the ones most readily involved in chemical bonding, determining an element's reactivity and the types of compounds it can form. They dictate the element's oxidation state, its tendency to gain, lose, or share electrons with other atoms. The number of valence electrons is primarily determined by an atom's position within the periodic table, specifically its group number (excluding transition metals).
Strontium's Position in the Periodic Table
Strontium resides in Group 2, also known as the alkaline earth metals, of the periodic table. This group's defining characteristic is that its elements all have two valence electrons. This consistent valence electron count significantly influences their chemical behavior, resulting in similar properties across the group.
Determining Strontium's Electronic Configuration
To definitively confirm strontium's valence electron count, we need to examine its electronic configuration. This configuration describes the arrangement of electrons in various energy levels and sublevels within the atom. Strontium's atomic number is 38, indicating that it has 38 electrons. Using the Aufbau principle and Hund's rule, we can determine its electronic configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²
This configuration reveals the distribution of strontium's 38 electrons across various energy levels. The key takeaway here lies in the outermost shell, the fifth energy level (n=5).
Identifying Strontium's Valence Electrons
The outermost shell, the 5s orbital, contains two electrons. These are strontium's valence electrons. They are the furthest from the nucleus and therefore experience the least attraction from the positive charge of the nucleus. This makes them the most readily available for participation in chemical bonding.
Visualizing Valence Electrons
Imagine the nucleus of a strontium atom as a central sun, and the electrons orbiting it like planets. The valence electrons reside in the outermost "orbit," making them easily accessible for interaction with other atoms. Their loose association with the nucleus explains strontium's reactivity.
Strontium's Chemical Behavior: A Consequence of its Valence Electrons
Strontium's two valence electrons are the key to understanding its chemical reactivity and the types of compounds it forms. Because achieving a stable electron configuration (like that of a noble gas) is energetically favorable, strontium readily loses these two valence electrons to form a +2 cation (Sr²⁺). This ionization process results in a stable electron configuration, similar to that of krypton (Kr).
Chemical Bonding in Strontium Compounds
This tendency to lose two electrons leads strontium to primarily form ionic bonds. In ionic bonding, strontium donates its two valence electrons to a more electronegative atom, such as chlorine (Cl), oxygen (O), or sulfur (S). This electron transfer generates oppositely charged ions (cations and anions) that are held together by electrostatic attraction, forming ionic compounds. Examples include strontium chloride (SrCl₂), strontium oxide (SrO), and strontium sulfide (SrS).
Oxidation State and Reactivity
The consistent loss of two electrons also determines strontium's common oxidation state of +2. This means that strontium almost always participates in chemical reactions by losing two electrons. This predictability makes strontium's chemical behavior relatively straightforward to understand and predict. Its high reactivity, especially with water and acids, is directly linked to its eagerness to lose its two valence electrons.
Applications of Strontium: The Role of Valence Electrons
The unique properties of strontium, stemming directly from its two valence electrons and its tendency to form ionic compounds, lead to its use in diverse applications:
1. Fireworks: A Colorful Display
Strontium's vibrant red color when burned is a well-known spectacle in fireworks. This intense red emission is a direct consequence of strontium's electronic structure. When strontium atoms are heated in a flame, their valence electrons absorb energy and transition to higher energy levels. As these electrons return to their ground state, they emit light at specific wavelengths, resulting in the characteristic red color. The intensity of the color is directly related to the concentration of strontium in the firework mixture.
2. Nuclear Medicine: Targeting Cancer Cells
Strontium-89, a radioactive isotope of strontium, is used in nuclear medicine for the palliative treatment of bone metastases (cancer spread to the bones). Because strontium readily substitutes for calcium in bones, strontium-89 targets cancerous bone lesions. Its beta radiation helps reduce pain and slow the progression of the disease. The isotope's half-life and its tendency to replace calcium are crucial for its effectiveness and safety in this medical application.
3. Magnet Manufacturing: High-Performance Materials
Strontium ferrites are used in the manufacture of magnets. These materials combine strontium's chemical properties with iron's magnetic properties to create strong, yet relatively inexpensive magnets. Their application ranges from small motors to larger industrial magnets. The structure and the magnetic properties of these ferrites are governed by the interactions of the ions formed by strontium and iron.
4. Other Applications
Beyond fireworks, nuclear medicine and magnets, strontium finds applications in various other fields, including:
- Alloying: Strontium is used to improve the properties of certain alloys, enhancing their strength and durability.
- Cathode Ray Tubes (CRTs): Historically, strontium was used in CRTs, though this application has diminished significantly with the advent of flat-screen technologies.
- Refining Sugar: Strontium compounds have been used in the refining of sugar, although more modern methods have largely replaced this practice.
Conclusion: Valence Electrons as the Key
The number of valence electrons is paramount in understanding an element's chemical behavior. Strontium, with its two valence electrons, consistently exhibits a +2 oxidation state, readily forming ionic compounds and displaying a high reactivity. This fundamental characteristic directly influences its applications, from the vivid colors in fireworks to its use in targeted cancer therapies and advanced materials. Understanding the electronic structure of elements is fundamental to comprehending their properties and applications across various fields of science and technology. Strontium’s unique chemistry, governed by its two valence electrons, continues to drive innovation and advancements across diverse industries. Further research into strontium’s properties and applications promises to unlock even more exciting possibilities in the future.
Latest Posts
Latest Posts
-
Is The Square Root Of 42 Rational Or Irrational
Apr 02, 2025
-
Round 45 To The Nearest 10
Apr 02, 2025
-
What Is The Length Of A Line Segment
Apr 02, 2025
-
What Is The Ocean Between North America And Europe
Apr 02, 2025
-
How Are Compounds And Mixtures Alike
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Sr . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
