How Many Valence Electrons Does Sulfer Have
Juapaving
Mar 15, 2025 · 6 min read
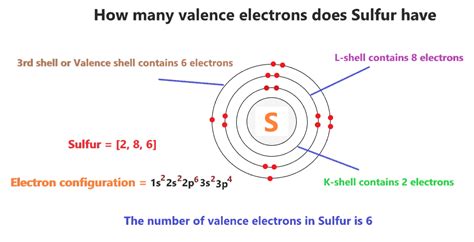
Table of Contents
How Many Valence Electrons Does Sulfur Have? A Deep Dive into Sulfur's Electronic Structure
Sulfur, a vibrant yellow nonmetal found abundantly in nature, plays a crucial role in various biological and industrial processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its remarkable chemical reactivity and diverse applications. This comprehensive guide will delve into the specifics of sulfur's valence electrons, exploring its electronic configuration, bonding behavior, and significance across different fields.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we pinpoint the number of valence electrons in sulfur, let's establish a fundamental understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of compounds it can form. They are the key players in the dance of chemical interactions, dictating how atoms interact to create molecules and compounds. Understanding valence electrons is essentially understanding the fundamental building blocks of chemistry.
Determining Sulfur's Valence Electrons: Electronic Configuration and the Octet Rule
Sulfur (S) has an atomic number of 16, meaning it possesses 16 protons and 16 electrons in a neutral atom. To determine its valence electrons, we need to examine its electronic configuration – the arrangement of electrons in its energy levels or shells. Using the Aufbau principle and Hund's rule, we can represent sulfur's electronic configuration as: 1s²2s²2p⁶3s²3p⁴.
This configuration tells us that sulfur has:
- Two electrons in the first shell (1s²)
- Eight electrons in the second shell (2s²2p⁶)
- Six electrons in the third shell (3s²3p⁴)
The outermost shell (the third shell) holds six electrons. These are sulfur's valence electrons.
Therefore, sulfur has six valence electrons.
This aligns with the octet rule, a fundamental principle in chemistry which states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration of eight electrons in their outermost shell (except for hydrogen and helium, which aim for two electrons). While sulfur doesn't always follow the octet rule perfectly (we'll explore exceptions later), its six valence electrons dictate its tendency to form bonds and achieve a more stable electron configuration.
Sulfur's Bonding Behavior: A Consequence of Six Valence Electrons
The presence of six valence electrons profoundly influences sulfur's bonding behavior. Sulfur can readily form covalent bonds by sharing electrons with other atoms, commonly forming molecules with other nonmetals. It can also participate in ionic bonding, gaining or losing electrons to achieve a stable octet, but this is less common than covalent bonding.
Here's a breakdown of sulfur's bonding tendencies:
Covalent Bonding:
- Sulfur's preference: Sulfur often shares two, four, or even six electrons to reach a stable octet, resulting in various bond orders.
- Examples: Hydrogen sulfide (H₂S) where sulfur shares two electrons, sulfur dioxide (SO₂) where sulfur shares four electrons, and sulfur hexafluoride (SF₆) where sulfur shares six electrons. The different bonding possibilities highlight sulfur's versatility in forming diverse compounds.
- Significance: Covalent bonding in sulfur leads to the formation of numerous essential molecules, including those crucial for biological functions.
Ionic Bonding (less common):
- Sulfide ion (S²⁻): In reactions with highly electropositive metals, sulfur can gain two electrons to form the sulfide ion, achieving a stable octet. This leads to the formation of ionic compounds such as sodium sulfide (Na₂S) and iron sulfide (FeS).
- Significance: Ionic bonding involving sulfur plays a role in mineral formation and geological processes.
The Significance of Sulfur and its Valence Electrons Across Various Fields
The unique properties stemming from sulfur's six valence electrons make it essential across a vast range of fields:
Biological Significance:
- Amino acids: Sulfur is a crucial component of two essential amino acids, cysteine and methionine. These amino acids play critical roles in protein structure and function, enzyme activity, and numerous metabolic processes.
- Enzymes: Sulfur-containing enzymes such as glutathione peroxidase (a vital antioxidant enzyme) and sulfhydryl oxidoreductases (involved in redox reactions) are essential for cellular health and protection.
- Vitamins: Sulfur is also present in biotin (vitamin B7), an important coenzyme involved in metabolism of carbohydrates, fats, and proteins.
Industrial Applications:
- Sulfuric acid (H₂SO₄): The most important industrial chemical, sulfuric acid's production relies on sulfur. It's widely used in fertilizer production, metal refining, oil refining, and numerous other industrial processes. Its versatility showcases the impact of sulfur's chemical reactivity.
- Rubber vulcanization: Sulfur plays a critical role in the vulcanization of rubber, enhancing its strength, durability, and elasticity. This is a classic example of the practical applications of sulfur's bonding capability.
- Other industrial applications: Sulfur and its compounds find applications in the production of detergents, pesticides, dyes, and various other materials, showcasing its wide-ranging impact on manufacturing.
Environmental Significance:
- Sulfur dioxide (SO₂): Released from volcanic eruptions and the burning of fossil fuels, sulfur dioxide is a significant air pollutant contributing to acid rain and respiratory problems. Understanding its chemical behavior, rooted in sulfur's valence electrons, is crucial for environmental protection and mitigation strategies.
- Sulfur in the atmosphere: Sulfur compounds in the atmosphere influence climate patterns and cloud formation, impacting global weather systems.
Exceptions to the Octet Rule: Sulfur's Versatility
While the octet rule serves as a useful guideline, sulfur can sometimes exceed the octet, showcasing its capacity to expand its valence shell. This is possible because sulfur has vacant d-orbitals in its third energy level, which can accommodate additional electrons. This ability allows sulfur to form compounds like sulfur hexafluoride (SF₆), where sulfur has twelve electrons around it (six covalent bonds). This is a classic example of sulfur exceeding the octet rule due to its expanded valence shell. This exception underscores the versatility and richness of sulfur's chemistry.
Conclusion: Sulfur's Six Valence Electrons – A Foundation for Diverse Applications
Sulfur's six valence electrons are the key to understanding its remarkable chemical behavior and its wide-ranging importance in biology, industry, and the environment. Its ability to form a variety of bonds, both covalent and ionic, and its capacity to exceed the octet rule, showcase its versatility and significance. From essential biological molecules to industrial processes and environmental concerns, sulfur's role is multifaceted and deeply intertwined with its unique electronic configuration and the properties derived from its six valence electrons. A comprehensive understanding of these aspects is vital for advancements in diverse fields, highlighting the fundamental importance of learning about the basic building blocks of matter. This deep dive into sulfur’s electronic structure underscores the intricate connection between atomic structure and macroscopic properties, emphasizing the power of fundamental chemistry in shaping our world.
Latest Posts
Latest Posts
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Sulfer Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
