How Many Valence Electrons Are In Potassium
Juapaving
Mar 26, 2025 · 5 min read
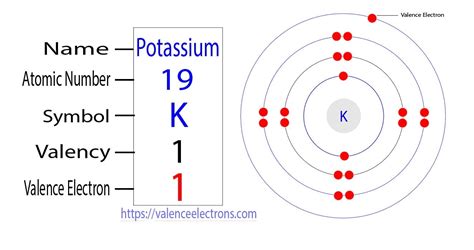
Table of Contents
How Many Valence Electrons Are in Potassium? A Deep Dive into Atomic Structure
Potassium, a crucial element for human health and a key player in various industrial applications, holds a fascinating place in the periodic table. Understanding its electronic configuration, particularly the number of valence electrons, is key to comprehending its reactivity and behavior. This comprehensive guide will delve into the details of potassium's atomic structure, explaining not just how many valence electrons it possesses but why this number is so significant.
Understanding Valence Electrons: The Key to Reactivity
Before we pinpoint the number of valence electrons in potassium, let's establish a fundamental understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the most loosely bound to the nucleus and, therefore, participate most readily in chemical bonding. The number of valence electrons dictates an atom's reactivity – its tendency to gain, lose, or share electrons to achieve a stable electron configuration, often following the octet rule (eight electrons in the outermost shell).
Potassium's Position in the Periodic Table: A Clue to its Valence Electrons
The periodic table is a powerful tool for predicting the properties of elements, including their valence electrons. Potassium (K), with an atomic number of 19, is located in Group 1 (also known as Alkali Metals) of the periodic table. The group number itself provides a strong hint regarding the number of valence electrons. Group 1 elements are characterized by having one valence electron.
Determining Potassium's Electron Configuration: The Foundation
To confirm the number of valence electrons in potassium, we need to determine its electron configuration. This describes how electrons are distributed among the different energy levels and subshells within the atom. For potassium, the electron configuration is:
1s²2s²2p⁶3s²3p⁶4s¹
Let's break this down:
- 1s²: Two electrons in the first energy level (n=1), in the s subshell.
- 2s²: Two electrons in the second energy level (n=2), in the s subshell.
- 2p⁶: Six electrons in the second energy level (n=2), in the p subshell.
- 3s²: Two electrons in the third energy level (n=3), in the s subshell.
- 3p⁶: Six electrons in the third energy level (n=3), in the p subshell.
- 4s¹: One electron in the fourth energy level (n=4), in the s subshell.
The outermost shell for potassium is the fourth energy level (n=4). This shell contains only one electron – the 4s¹ electron. Therefore, potassium has one valence electron.
Why the Single Valence Electron Matters: Reactivity and Chemical Bonding
Potassium's single valence electron is the key to understanding its high reactivity. To achieve a stable octet configuration (like the noble gas Argon), potassium readily loses this single electron, forming a +1 ion (K⁺). This loss of an electron requires relatively little energy, making potassium a highly reactive element. This reactivity is evident in potassium's vigorous reaction with water, producing hydrogen gas and potassium hydroxide.
Potassium's Reactions: A Manifestation of its Valence Electron
The single valence electron allows potassium to participate in various chemical reactions, including:
- Reaction with water: Potassium reacts violently with water, generating hydrogen gas and heat. This reaction is a direct consequence of potassium's eagerness to lose its valence electron.
- Formation of ionic compounds: Potassium readily forms ionic compounds with nonmetals, such as chlorine (forming potassium chloride, KCl) and oxygen (forming potassium oxide, K₂O). In these compounds, potassium loses its valence electron to achieve a stable octet, while the nonmetal gains the electron.
- Formation of alloys: Potassium can also form alloys with other metals, further illustrating its tendency to interact with other elements through electron transfer.
Applications of Potassium: A Reflection of its Properties
The unique properties stemming from potassium's single valence electron translate into a range of important applications:
- Agriculture: Potassium is a vital macronutrient for plant growth, playing a crucial role in various metabolic processes. Potassium fertilizers are widely used to enhance crop yields and improve plant health.
- Medicine: Potassium ions (K⁺) are essential for maintaining proper electrolyte balance in the human body. They are involved in nerve impulse transmission, muscle contraction, and maintaining a healthy heartbeat. Potassium deficiencies can lead to serious health problems.
- Industry: Potassium compounds are used in various industrial applications, including the production of soaps, glass, and fertilizers. Potassium hydroxide (KOH) is a strong base used in numerous industrial processes.
Comparing Potassium to Other Alkali Metals: Trends in Valence Electrons
Potassium belongs to the alkali metal group, which also includes lithium (Li), sodium (Na), rubidium (Rb), cesium (Cs), and francium (Fr). All alkali metals share the common characteristic of having one valence electron. However, there are subtle differences in their reactivity. As you move down the group (increasing atomic number), the reactivity generally increases. This is because the outermost electron is further from the nucleus and thus more easily lost. Therefore, potassium is more reactive than lithium and sodium but less reactive than rubidium and cesium.
Beyond the Single Valence Electron: A Deeper Look at Potassium's Atomic Structure
While the single valence electron dominates potassium's chemical behavior, it's crucial to appreciate the role of its inner electrons and the overall atomic structure. The inner electrons shield the valence electron from the full positive charge of the nucleus, influencing the ease with which the valence electron can be lost. The size of the atom, determined by the number of electron shells, also plays a role in reactivity.
Conclusion: The Significance of Potassium's Single Valence Electron
In conclusion, potassium possesses one valence electron, a fact that dictates its chemical behavior and numerous applications. This single electron is the key to its high reactivity, its ability to form ionic compounds, and its importance in biological systems and industrial processes. Understanding the valence electron configuration is crucial for comprehending the properties and behavior of elements, and potassium serves as an excellent example of how this fundamental concept governs the world around us. From the fields of agriculture to medicine and industrial chemistry, the single valence electron in potassium profoundly influences our lives.
Latest Posts
Latest Posts
-
Which Is Larger 3 8 Or 1 2
Mar 27, 2025
-
Which Of The Following Are Not Trigonometric Identities
Mar 27, 2025
-
Why Are Most Ionic Substances Brittle
Mar 27, 2025
-
What Are Examples Of Unit Rates
Mar 27, 2025
-
What Is The Least Common Multiple Of 18 And 6
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
