How Many Valence Electrons Are In H2o
Juapaving
Mar 31, 2025 · 6 min read
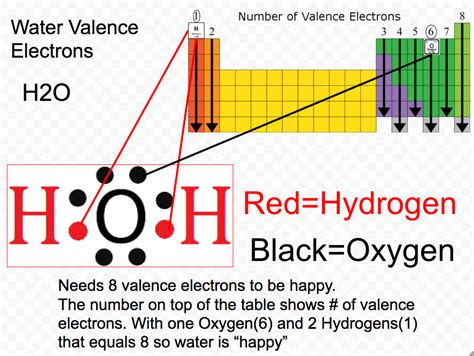
Table of Contents
How Many Valence Electrons Are in H₂O? A Deep Dive into Water's Electronic Structure
Water, H₂O, is a seemingly simple molecule, yet its unique properties are fundamental to life as we know it. Understanding its electronic structure, particularly the number of valence electrons, is key to grasping these properties. This article will delve deep into the electronic configuration of water, explaining not only the answer to the central question – how many valence electrons are in H₂O? – but also the implications of this electron count for water's behavior.
Understanding Valence Electrons
Before diving into the specifics of H₂O, let's establish a solid understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they determine how an atom will interact with other atoms to form chemical bonds. They're the primary players in chemical reactions, dictating an element's reactivity and the types of bonds it can form.
The number of valence electrons an atom possesses is determined by its position in the periodic table. Specifically, it's related to the atom's group number (vertical columns). For example, elements in Group 1 (alkali metals) have one valence electron, those in Group 2 (alkaline earth metals) have two, and so on. However, this pattern becomes slightly more complex for transition metals (d-block elements) and post-transition metals (p-block elements).
Determining Valence Electrons for Hydrogen and Oxygen
Let's apply this to the atoms in water: hydrogen (H) and oxygen (O).
-
Hydrogen (H): Hydrogen is located in Group 1 of the periodic table, meaning it has one valence electron. Its electron configuration is 1s¹.
-
Oxygen (O): Oxygen is located in Group 16 (or VIA) of the periodic table, meaning it has six valence electrons. Its electron configuration is 1s²2s²2p⁴. The 2s and 2p orbitals are the outermost shells, and they contain a total of six electrons.
Calculating Valence Electrons in H₂O
Now that we know the number of valence electrons for each atom, we can calculate the total number for the H₂O molecule.
Water (H₂O) consists of two hydrogen atoms and one oxygen atom. Therefore, the total number of valence electrons in H₂O is:
(2 Hydrogen atoms x 1 valence electron/atom) + (1 Oxygen atom x 6 valence electrons/atom) = 2 + 6 = 8 valence electrons
Therefore, the answer to our central question is: There are 8 valence electrons in a water molecule (H₂O).
The Role of Valence Electrons in Water's Properties
The eight valence electrons in H₂O are not distributed uniformly. The oxygen atom, being more electronegative than hydrogen, attracts the shared electrons more strongly. This unequal sharing of electrons leads to the formation of polar covalent bonds. Each O-H bond is a covalent bond (electrons are shared), but the electrons are pulled closer to the oxygen atom, creating a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens.
This polarity is crucial to many of water's unique properties, including:
1. High Boiling Point:
The strong hydrogen bonds between water molecules, a consequence of the molecule's polarity, require significant energy to break. This results in water's relatively high boiling point compared to other molecules of similar size.
2. Excellent Solvent:
Water's polarity makes it an excellent solvent for many ionic and polar substances. The partial charges on the water molecule can interact with and surround ions or polar molecules, dissolving them in the process. This is crucial for biological processes, as water acts as a solvent for many vital biological molecules.
3. High Surface Tension:
The hydrogen bonds between water molecules create a strong cohesive force, leading to a high surface tension. This allows insects to walk on water and contributes to the capillary action that moves water through plants.
4. High Specific Heat Capacity:
Water has a high specific heat capacity, meaning it can absorb a large amount of heat energy without a significant change in temperature. This helps regulate temperature fluctuations in aquatic environments and within living organisms.
5. Density Anomaly:
Ice is less dense than liquid water, a unique property that has significant implications for aquatic life. This anomaly is due to the specific arrangement of hydrogen bonds in the ice crystal structure.
Beyond the Basic Calculation: A Deeper Look at Molecular Orbital Theory
While the simple valence electron count provides a good starting point, a more complete understanding of H₂O's electronic structure requires considering molecular orbital theory. This theory describes how atomic orbitals combine to form molecular orbitals, which are regions of space where electrons are likely to be found in a molecule.
In H₂O, the oxygen atom's 2s and 2p orbitals hybridize to form four sp³ hybrid orbitals. Two of these sp³ orbitals overlap with the hydrogen 1s orbitals to form two sigma (σ) bonds, representing the two O-H bonds. The remaining two sp³ orbitals contain lone pairs of electrons, contributing significantly to the molecule's bent geometry and polarity.
The molecular orbital diagram for H₂O shows the distribution of electrons in bonding and antibonding orbitals. The filling of these orbitals further explains the molecule's stability and properties. This level of analysis goes beyond simply counting valence electrons but provides a richer picture of the electronic interactions within the molecule.
Applications and Further Exploration
Understanding the valence electron configuration of H₂O is not just an academic exercise. It has practical applications in many fields, including:
- Chemistry: Understanding the bonding and reactivity of water is essential for predicting the outcome of chemical reactions involving water.
- Biology: The properties of water are fundamental to life, influencing biological processes such as protein folding, enzyme activity, and membrane transport.
- Environmental Science: Water's properties are crucial for understanding hydrological cycles, climate change impacts, and water pollution.
- Materials Science: The properties of water are important in many materials processing applications, such as the design of new materials with specific properties.
Further exploration into the electronic structure of water could involve investigating:
- Excited states: What happens when water absorbs energy and electrons are promoted to higher energy levels?
- Spectroscopic techniques: How can we use spectroscopic techniques to experimentally probe the electronic structure of water?
- Computational chemistry: How can we use computational methods to model and simulate the electronic structure and properties of water?
Conclusion
The seemingly simple question of how many valence electrons are in H₂O leads to a fascinating exploration of the molecule's electronic structure and its profound impact on the world around us. Understanding the eight valence electrons and their distribution is fundamental to grasping water's unique properties, which are essential to numerous natural processes and technological applications. This exploration serves as a stepping stone to a deeper understanding of chemical bonding, molecular structure, and the intricate relationship between electronic configuration and macroscopic properties. By exploring this seemingly simple molecule, we unveil a world of complexity and vital significance.
Latest Posts
Latest Posts
-
Which Base Is Not Present In Rna
Apr 02, 2025
-
What Is The Lcm Of 5 And 10
Apr 02, 2025
-
Air Moves From High To Low Pressure
Apr 02, 2025
-
The Smallest Particle Of An Element Is A N
Apr 02, 2025
-
How Many Neutrons Does A Hydrogen Atom Have
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
