How Many Protons Electrons And Neutrons Are In Sodium
Juapaving
Mar 16, 2025 · 5 min read
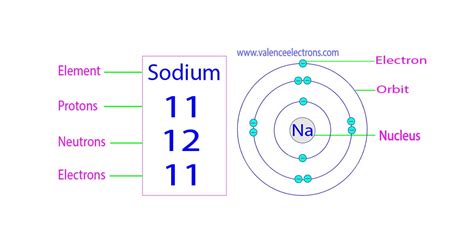
Table of Contents
How Many Protons, Electrons, and Neutrons are in Sodium? A Deep Dive into Atomic Structure
Sodium, a ubiquitous element crucial for life and various industrial applications, presents a fascinating case study in understanding atomic structure. This article delves deep into the composition of a sodium atom, exploring the numbers of protons, electrons, and neutrons it possesses, and how this composition relates to its chemical properties and behavior. We'll also touch upon isotopes and their impact on the overall composition.
Understanding the Basics: Protons, Electrons, and Neutrons
Before we dive into the specifics of sodium, let's establish a foundational understanding of the subatomic particles that constitute an atom:
Protons: The Positive Charge Carriers
Protons are positively charged particles residing within the atom's nucleus. The number of protons defines the element itself. This number is known as the atomic number. Every atom of a given element will have the same number of protons. Changes in proton number fundamentally alter the element's identity.
Electrons: The Negative Charge Carriers
Electrons are negatively charged particles that orbit the nucleus at various energy levels or shells. Unlike protons, the number of electrons in an atom can vary, leading to the formation of ions. In a neutral atom, the number of electrons equals the number of protons, resulting in a net charge of zero.
Neutrons: The Neutral Particles
Neutrons are neutrally charged particles, also residing in the atom's nucleus. They contribute to the atom's mass but don't affect its charge. The number of neutrons can vary within the same element, leading to the existence of isotopes.
Sodium's Atomic Structure: Unpacking the Numbers
Now, let's focus on sodium (Na), an element with an atomic number of 11. This atomic number signifies that every sodium atom contains 11 protons. This is a defining characteristic of sodium and distinguishes it from all other elements.
Because a neutral sodium atom has an equal number of protons and electrons to maintain a neutral charge, it also possesses 11 electrons. These electrons are arranged in three electron shells: two in the first shell, eight in the second, and one in the outermost, or valence, shell. This single valence electron is crucial in understanding sodium's chemical reactivity.
The number of neutrons, however, is slightly more complex. Most sodium atoms have 12 neutrons, giving them a mass number of 23 (protons + neutrons = 11 + 12 = 23). This is the most abundant isotope of sodium, often represented as ²³Na.
Isotopes: Variations in Neutron Number
While the most common sodium isotope has 12 neutrons, it's important to remember that isotopes exist. Isotopes are atoms of the same element (same number of protons) but with differing numbers of neutrons. This means their mass numbers vary.
Sodium has several known isotopes, including:
- ²²Na: This isotope has 11 protons and 11 neutrons. It's radioactive.
- ²³Na: This is the most abundant and stable isotope of sodium, with 11 protons and 12 neutrons.
- ²⁴Na: This isotope has 11 protons and 13 neutrons and is radioactive.
The presence of isotopes affects the average atomic mass of sodium reported on the periodic table. The average atomic mass takes into account the abundance of each isotope in naturally occurring sodium.
The Significance of Sodium's Electronic Configuration
The presence of a single electron in the outermost shell is what dictates sodium's chemical behavior. This electron is easily lost, making sodium highly reactive. It readily loses this electron to achieve a stable electron configuration, similar to the noble gas neon. This process forms a positively charged sodium ion (Na⁺).
This tendency to lose an electron and form a positive ion is crucial in understanding sodium's role in various chemical reactions and its importance in biological systems. Sodium ions are essential for nerve impulse transmission, muscle contraction, and fluid balance in living organisms.
Sodium in Everyday Life and Industrial Applications
Sodium's unique properties due to its electronic configuration make it essential in various applications:
Biological Importance:
- Nerve Impulse Transmission: Sodium ions play a vital role in generating and transmitting nerve impulses. The movement of sodium ions across cell membranes is crucial for the communication between nerve cells.
- Muscle Contraction: Similar to nerve impulse transmission, sodium ions are essential for muscle contraction. Changes in sodium ion concentrations across muscle cell membranes trigger muscle contraction.
- Fluid Balance: Sodium ions contribute significantly to maintaining the proper balance of fluids within the body.
Industrial Applications:
- Sodium Lamps: Sodium vapor lamps produce a characteristic bright yellow light, widely used in street lighting and other outdoor illumination.
- Sodium Chloride (Table Salt): Sodium chloride, a compound of sodium and chlorine, is a fundamental seasoning agent and preservative in food. Its importance in human diet is well-established.
- Sodium Hydroxide (Caustic Soda): This strong base has a broad range of industrial applications, including soap making, paper production, and drain cleaning.
- Sodium Carbonate (Washing Soda): Used as a water softener and cleaning agent.
Conclusion: Understanding Sodium's Atomic Structure – A Key to Understanding its Properties
Understanding the precise number of protons, electrons, and neutrons in sodium – 11, 11, and typically 12, respectively – is critical to grasping its chemical properties and reactivity. The presence of a single valence electron leads to its readiness to lose an electron and form a positive ion, explaining its significance in biological processes and industrial applications. The existence of isotopes further enhances our understanding of the element's variations in mass and its average atomic weight as seen on the periodic table. Therefore, the seemingly simple atomic structure of sodium unveils a world of complexity and significance across diverse fields. The interplay between subatomic particles determines its remarkable properties, highlighting the importance of studying atomic structure to understand the macroscopic properties and behavior of elements. Further research into the radioactive isotopes can provide further insights into nuclear processes and their applications in medicine and other scientific fields.
Latest Posts
Latest Posts
-
What Is An Operator In Biology
Mar 17, 2025
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Electrons And Neutrons Are In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
