How Many Protons And Neutrons Does Potassium Have
Juapaving
Mar 21, 2025 · 5 min read
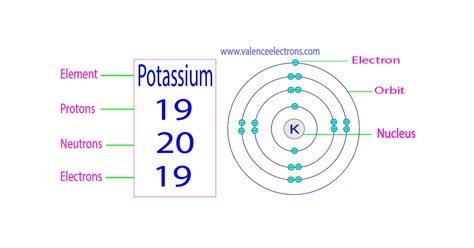
Table of Contents
How Many Protons and Neutrons Does Potassium Have? A Deep Dive into Atomic Structure
Potassium, a vital element for life, plays a crucial role in numerous biological processes. Understanding its atomic structure, specifically the number of protons and neutrons, is fundamental to grasping its chemical behavior and biological significance. This in-depth article will explore the atomic composition of potassium, delve into isotopic variations, and discuss the implications of this atomic structure in various contexts.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the specifics of potassium, let's establish a foundational understanding of atomic structure. An atom comprises three subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and determines its chemical identity. No two elements have the same number of protons.
-
Neutrons: Electrically neutral particles also found in the nucleus. They contribute to the atom's mass but not its charge. The number of neutrons can vary within an element, leading to isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom. However, atoms can gain or lose electrons, forming ions with a net positive or negative charge.
Potassium's Atomic Number and Proton Count
Potassium (K), located in Group 1 (alkali metals) of the periodic table, has an atomic number of 19. This unequivocally means that every potassium atom possesses 19 protons. This fundamental characteristic dictates potassium's chemical properties and its position within the periodic table. The arrangement of these 19 protons within the nucleus dictates its stability and reactivity.
Neutrons in Potassium: The Role of Isotopes
Unlike the fixed number of protons, the number of neutrons in potassium can vary. Atoms of the same element with differing neutron counts are called isotopes. These isotopes exhibit similar chemical behavior due to their identical number of protons and electrons, but their physical properties (like mass) differ slightly.
Potassium has several naturally occurring isotopes, the most prevalent being:
-
Potassium-39 (³⁹K): This isotope constitutes approximately 93.3% of naturally occurring potassium. It contains 19 protons and 20 neutrons (19 + 20 = 39). This is the most stable isotope of potassium.
-
Potassium-40 (⁴⁰K): This isotope is radioactive and makes up about 0.012% of naturally occurring potassium. It contains 19 protons and 21 neutrons (19 + 21 = 40). While radioactive, its long half-life (1.25 billion years) means it poses a relatively low risk. ⁴⁰K decays through both beta-plus and beta-minus decay, making it significant in geological dating.
-
Potassium-41 (⁴¹K): This stable isotope accounts for roughly 6.7% of naturally occurring potassium. It has 19 protons and 22 neutrons (19 + 22 = 41).
The Significance of Potassium's Atomic Structure in Biological Systems
The unique atomic structure of potassium, particularly its single electron in its outermost shell, makes it highly reactive. This reactivity is crucial for its biological functions:
-
Electrolyte Balance: Potassium is a vital electrolyte, playing a critical role in maintaining the proper balance of fluids and electrolytes within the body. Its ionic form (K⁺) participates in nerve impulse transmission and muscle contraction.
-
Enzyme Activation: Many enzymes require potassium ions for their proper function. Potassium's presence is essential for various metabolic processes.
-
Osmotic Regulation: Potassium contributes to maintaining osmotic pressure within cells, ensuring proper cellular hydration and function.
-
Blood Pressure Regulation: Potassium plays a crucial role in regulating blood pressure. Imbalances in potassium levels can lead to cardiovascular problems.
-
Nutrient Uptake: Potassium is vital for plant growth and development, aiding in nutrient uptake and overall plant health.
The isotopic composition of potassium, while subtly influencing its overall mass, doesn't drastically alter its biological roles. The majority of biological effects are governed by potassium's chemical properties, determined by its 19 protons and its tendency to lose one electron to become a stable K⁺ ion.
Potassium-40: Radioactive Decay and Implications
Potassium-40, the radioactive isotope, deserves further attention. Its decay produces beta particles and gamma radiation. While the radiation levels are relatively low, its presence has significant implications:
-
Geological Dating: The decay rate of ⁴⁰K is used in radiometric dating to determine the age of rocks and minerals. This is a cornerstone technique in geological studies.
-
Background Radiation: ⁴⁰K contributes to the natural background radiation we're all exposed to. Its contribution, while generally harmless, needs consideration in radiation safety assessments.
-
Medical Imaging: The decay of ⁴⁰K, though generally not used directly for medical imaging, can contribute slightly to the background noise in certain imaging techniques.
Beyond the Basics: Isotope Abundance and Applications
The relative abundance of potassium isotopes (³⁹K, ⁴⁰K, and ⁴¹K) varies slightly depending on the source material. These variations are minute but can be detected through sophisticated techniques like mass spectrometry. Such measurements have applications in:
-
Tracing Geological Processes: Isotopic analysis can reveal information about the geological history of a sample, including its origin and formation processes.
-
Environmental Studies: Isotopic variations in potassium can be used to trace the movement of water and nutrients in ecological systems.
-
Forensic Science: In some limited applications, isotopic analysis could potentially assist in forensic investigations.
Conclusion: Potassium's Atomic Structure and its Broad Significance
The simple answer to "How many protons and neutrons does potassium have?" is multifaceted. While all potassium atoms possess 19 protons, the neutron count varies among its isotopes. The most abundant isotope, ³⁹K, has 20 neutrons, while the radioactive isotope ⁴⁰K has 21 neutrons. Understanding this atomic structure is crucial for comprehending potassium's chemical behavior, its biological significance in various life forms, and its applications in diverse scientific fields. The seemingly straightforward question of proton and neutron count opens a door to a broader appreciation of the complexities of atomic structure, isotopic variation, and the essential roles of this element in our world. From maintaining electrolyte balance in our bodies to aiding geological dating, potassium's atomic characteristics underscore its widespread importance.
Latest Posts
Latest Posts
-
What Are All The Factors Of 80
Mar 28, 2025
-
Difference Between Current Electricity And Static Electricity
Mar 28, 2025
-
Salt A Commonly Used In Bakery Products
Mar 28, 2025
-
The Shapes Of The Horizontal Cross Sections
Mar 28, 2025
-
The Largest Gland Of The Body
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons And Neutrons Does Potassium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
