How Many Electrons In A Double Bond
Juapaving
Mar 18, 2025 · 6 min read
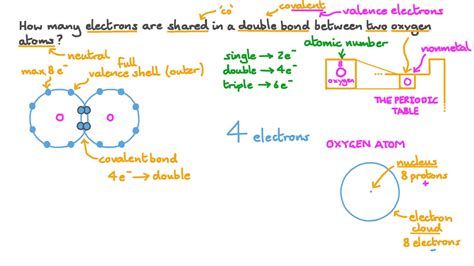
Table of Contents
How Many Electrons in a Double Bond? A Deep Dive into Chemical Bonding
Understanding chemical bonds is fundamental to grasping the behavior of matter. Among the various types of bonds, double bonds hold a significant place, exhibiting unique properties and influencing molecular structure and reactivity. This article will delve into the intricacies of double bonds, specifically addressing the central question: how many electrons are involved in a double bond? We'll explore the underlying principles of covalent bonding, delve into the formation of double bonds, examine examples, and discuss the implications of this type of bonding.
The Basics of Covalent Bonding
Before we tackle double bonds, it's crucial to understand the foundation of covalent bonding. Covalent bonds are formed when two atoms share one or more pairs of electrons. This sharing occurs because atoms strive to achieve a stable electron configuration, often resembling that of a noble gas with a full outer electron shell (octet rule). This sharing allows both atoms to effectively "fill" their outermost electron shells.
A single covalent bond involves the sharing of one pair of electrons, or two electrons. For instance, in a molecule of hydrogen (H₂), each hydrogen atom contributes one electron to form a single covalent bond, resulting in both atoms having a stable duet (two electrons) in their outermost shell. Similarly, in a molecule of methane (CH₄), carbon shares one electron with each of the four hydrogen atoms, forming four single covalent bonds.
Double Bonds: A Shared Quartet
Now, let's move to the focus of our discussion: double bonds. A double bond is formed when two atoms share two pairs of electrons, or a total of four electrons. This means each atom contributes two electrons to the bond. This results in a stronger bond than a single bond, due to the increased electron density between the bonded atoms.
Formation of a Double Bond
The formation of a double bond is driven by the same principle as a single bond: the desire for atoms to achieve a stable electron configuration. However, in a double bond, atoms achieve this stability by sharing a greater number of electrons. Let's consider the example of ethene (C₂H₄), also known as ethylene.
Each carbon atom in ethene has four valence electrons. To achieve a stable octet, each carbon atom needs to share four electrons. In ethene, each carbon atom shares two electrons with the other carbon atom to form a double bond (four electrons total), and one electron each with two hydrogen atoms to form two single bonds (two electrons total for each carbon). This arrangement allows both carbon atoms to have a full octet, fulfilling the octet rule.
Key Points about Double Bond Formation:
- Two pairs of electrons: The defining characteristic of a double bond.
- Stronger than single bonds: The increased electron density leads to higher bond strength and shorter bond length.
- Planar geometry: Atoms involved in a double bond often exhibit planar geometry due to the presence of p-orbitals involved in the pi bond.
- Restricted rotation: The double bond restricts rotation around the bond axis compared to a single bond. This rigidity plays a critical role in the shape and properties of molecules.
Examples of Double Bonds in Organic Molecules
Double bonds are prevalent in organic chemistry, forming the backbone of many important molecules. Some prominent examples include:
- Alkenes: These hydrocarbons contain at least one carbon-carbon double bond. Examples include ethene (C₂H₄), propene (C₃H₆), and butene (C₄H₈).
- Carbonyl groups: These functional groups consist of a carbon atom double-bonded to an oxygen atom (C=O). They are found in aldehydes, ketones, carboxylic acids, and esters. The carbonyl group's double bond significantly impacts the reactivity of these molecules.
- Carbon dioxide (CO₂): This simple molecule consists of a carbon atom double-bonded to two oxygen atoms, with each double bond consisting of four electrons.
- Benzene (C₆H₆): This aromatic hydrocarbon contains a ring structure with alternating single and double bonds between carbon atoms. However, it's crucial to note that the electron distribution in benzene is delocalized, implying a resonance structure rather than fixed single and double bonds. This delocalization results in exceptional stability.
Double Bonds vs. Single and Triple Bonds
To further solidify understanding, let's contrast double bonds with single and triple bonds:
| Bond Type | Number of Electron Pairs | Number of Electrons | Bond Strength | Bond Length |
|---|---|---|---|---|
| Single Bond | 1 | 2 | Weakest | Longest |
| Double Bond | 2 | 4 | Stronger than single | Shorter than single |
| Triple Bond | 3 | 6 | Strongest | Shortest |
Implications of Double Bonds
The presence of a double bond significantly impacts the properties and reactivity of molecules. Several key implications include:
- Higher bond energy: Double bonds require more energy to break compared to single bonds, contributing to their increased stability.
- Shorter bond length: The greater electron density pulls the atoms closer together, resulting in a shorter bond length.
- Planar geometry (often): Double bonds often restrict the movement around the bond axis, leading to planar or near-planar geometry in molecules.
- Reactivity: Double bonds are more reactive than single bonds due to the presence of pi electrons, which are more readily available for reactions. This is a crucial factor in many chemical reactions, such as addition reactions.
Delocalized Electrons and Resonance Structures
In certain molecules, electrons in double bonds aren't confined to a single location between two atoms. Instead, they are delocalized across multiple atoms, resulting in resonance structures. Benzene is a classic example, as mentioned earlier. The delocalized electrons contribute to the stability and unique properties of these molecules.
Beyond Organic Chemistry: Double Bonds in Inorganic Compounds
While the examples above primarily focus on organic molecules, double bonds are not limited to carbon-based compounds. Many inorganic compounds also exhibit double bonds. For instance, carbon dioxide (CO₂) features two carbon-oxygen double bonds, while sulfur dioxide (SO₂) has a sulfur-oxygen double bond and a sulfur-oxygen single bond (with resonance structures). These double bonds in inorganic molecules play a crucial role in their chemical and physical properties.
Advanced Concepts: Sigma and Pi Bonds
To further delve into the nature of double bonds, we introduce the concepts of sigma (σ) and pi (π) bonds. A double bond consists of one sigma bond and one pi bond.
- Sigma (σ) bond: This is a strong, single bond formed by the direct overlap of atomic orbitals along the internuclear axis. It's the first bond formed in a double or triple bond.
- Pi (π) bond: This weaker bond is formed by the sideways overlap of p-orbitals above and below the internuclear axis. It's formed after the sigma bond in a multiple bond.
The presence of the pi bond contributes to the unique reactivity and properties of double bonds.
Conclusion: The Significance of Four Electrons
In conclusion, a double bond involves the sharing of four electrons between two atoms, formed by two pairs of electrons. This seemingly simple fact underpins the intricate chemistry of countless molecules, impacting their structure, reactivity, and properties. From the simplest alkenes to complex biological molecules, the double bond plays an indispensable role in the world around us. Understanding this fundamental aspect of chemical bonding is essential for anyone seeking a deeper understanding of chemistry. This comprehensive overview provides a robust foundation for further exploration into the fascinating world of chemical bonding and molecular structure.
Latest Posts
Latest Posts
-
A Generator Is A Device That Converts Energy Into Energy
Mar 19, 2025
-
What Is The Conservation Of Charge
Mar 19, 2025
-
How Many Faces On A Dice
Mar 19, 2025
-
Which Kingdom Does Not Contain Any Eukaryotes
Mar 19, 2025
-
Database Is A Collection Of Related Data
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons In A Double Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
