How Many Electrons Can 4f Hold
Juapaving
Mar 30, 2025 · 6 min read
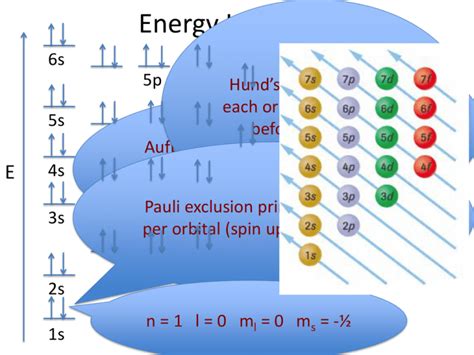
Table of Contents
How Many Electrons Can a 4f Subshell Hold? A Deep Dive into Electron Configuration
The question of how many electrons a 4f subshell can hold is fundamental to understanding atomic structure and electron configuration. While the answer is relatively straightforward, a deeper exploration reveals fascinating insights into quantum mechanics and the periodic table. This article will not only answer the question directly but delve into the underlying principles governing electron arrangement within atoms.
Understanding Electron Shells, Subshells, and Orbitals
Before tackling the 4f subshell specifically, let's establish a foundational understanding of atomic structure. Electrons, negatively charged particles, orbit the nucleus of an atom in distinct energy levels called shells. These shells are further divided into subshells, which are identified by the letters s, p, d, and f. Each subshell contains one or more orbitals, regions of space where there's a high probability of finding an electron.
-
Shells (n): Designated by the principal quantum number (n), representing the energy level. n can be any positive integer (1, 2, 3, etc.). Higher n values indicate higher energy levels and greater distance from the nucleus.
-
Subshells (l): Designated by the azimuthal quantum number (l), which determines the shape of the orbital and its angular momentum. l can range from 0 to n-1. This translates to:
- l = 0: s subshell (spherical shape, 1 orbital)
- l = 1: p subshell (dumbbell shape, 3 orbitals)
- l = 2: d subshell (more complex shapes, 5 orbitals)
- l = 3: f subshell (even more complex shapes, 7 orbitals)
-
Orbitals (m<sub>l</sub>): Designated by the magnetic quantum number (m<sub>l</sub>), which specifies the orientation of the orbital in space. m<sub>l</sub> can range from -l to +l, including 0. This means:
- s subshell: 1 orbital (m<sub>l</sub> = 0)
- p subshell: 3 orbitals (m<sub>l</sub> = -1, 0, +1)
- d subshell: 5 orbitals (m<sub>l</sub> = -2, -1, 0, +1, +2)
- f subshell: 7 orbitals (m<sub>l</sub> = -3, -2, -1, 0, +1, +2, +3)
The Pauli Exclusion Principle: A Crucial Rule
The Pauli Exclusion Principle is paramount to understanding electron configuration. This principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, m<sub>l</sub>, and m<sub>s</sub>). The spin quantum number (m<sub>s</sub>) describes the intrinsic angular momentum of an electron, with two possible values: +1/2 (spin up) and -1/2 (spin down).
This principle dictates that each orbital can hold a maximum of two electrons, one with spin up and one with spin down. This is crucial when determining the maximum number of electrons a subshell can accommodate.
How Many Electrons Can the 4f Subshell Hold?
Now, let's finally answer the central question. Since the 4f subshell contains 7 orbitals, and each orbital can hold a maximum of 2 electrons according to the Pauli Exclusion Principle, the 4f subshell can hold a total of 14 electrons.
Visualizing the 4f Subshell
Imagine seven distinct, complex-shaped regions of space within the atom. Each region can accommodate a pair of electrons with opposite spins. Filling the 4f subshell means populating each of these seven orbitals with two electrons each, resulting in a total of 14 electrons.
The 4f Subshell and the Lanthanides
The 4f subshell plays a critical role in the properties of the lanthanides, also known as the rare earth elements. These elements are characterized by the progressive filling of the 4f subshell. As you move across the lanthanide series in the periodic table, electrons sequentially fill the 4f orbitals. This gradual filling of the inner 4f subshell leads to the similar chemical properties observed within this group of elements.
Electron Configuration and the Aufbau Principle
The order in which electrons fill the subshells is not simply a matter of filling the lowest energy levels first. The Aufbau principle, or building-up principle, guides this process. While generally following the increasing energy levels, there are exceptions due to subtle energy differences between subshells.
The general filling order is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... and so on. Note the irregularities; this is a simplified representation, and the actual filling order can be influenced by electron-electron interactions.
Understanding this filling order is essential for predicting electron configurations of different atoms. For instance, the element Gadolinium (Gd, atomic number 64) has an electron configuration that includes a fully filled 4f subshell with all 14 electrons.
Implications of 4f Electron Configuration
The electron configuration, and specifically the filling of the 4f subshell, significantly impacts the physical and chemical properties of elements.
-
Magnetic Properties: The unpaired electrons in the 4f subshell contribute to the unique magnetic properties exhibited by many lanthanides. This is exploited in various applications, including magnetic resonance imaging (MRI) and certain types of magnets.
-
Chemical Reactivity: The shielding effect of the 4f electrons influences the reactivity of lanthanides. The relatively poor shielding of the 4f electrons can lead to variations in chemical behavior compared to elements in other periods.
-
Spectroscopic Properties: The transitions of electrons within the 4f subshell give rise to characteristic absorption and emission spectra, useful in analytical chemistry for identifying lanthanide elements.
-
Catalytic Activity: The partially filled 4f subshell in some lanthanides contributes to their catalytic activity in various chemical reactions. They are used as catalysts in various industrial processes.
Beyond the Basics: Advanced Concepts
The simple model of electron shells and subshells provides a good starting point, but a complete understanding requires exploring more advanced quantum mechanical concepts. For example:
-
Electron Correlation: The interactions between electrons are not perfectly accounted for in the simple model. These interactions influence the energy levels and thus the electron configuration.
-
Relativistic Effects: At higher atomic numbers, relativistic effects become more significant, influencing the energies of electrons in inner subshells, including the 4f subshell.
-
Quantum Electrodynamics (QED): QED offers a more precise description of the interaction between electrons and the electromagnetic field, refining our understanding of electron behavior.
Conclusion
The 4f subshell, with its capacity to hold 14 electrons, is a vital component in understanding atomic structure and the periodic table. Its significance extends far beyond a simple numerical answer, influencing the physical and chemical properties of the lanthanide series and impacting various technological applications. This exploration has not only answered the central question but also provided a deeper appreciation for the intricate world of quantum mechanics and its profound consequences on the behavior of matter. Further exploration into the advanced concepts mentioned above will provide even greater insight into the fascinating world of atomic structure.
Latest Posts
Latest Posts
-
What Is The Ultimate Source Of Energy In Ecosystem
Apr 01, 2025
-
What Shape Has 6 Faces 8 Vertices And 12 Edges
Apr 01, 2025
-
5 Letter Words Start With Vi
Apr 01, 2025
-
Which Of The Following Is An Exothermic Reaction
Apr 01, 2025
-
Area Moment Of Inertia Of A Triangle
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can 4f Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
