Balanced Equation For Sodium Carbonate And Hydrochloric Acid
Juapaving
Mar 17, 2025 · 5 min read
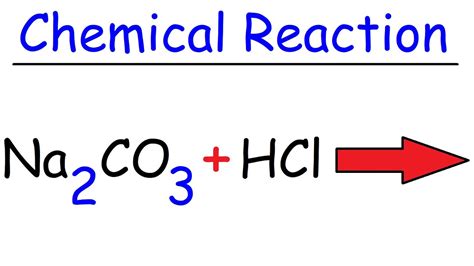
Table of Contents
The Balanced Equation for Sodium Carbonate and Hydrochloric Acid: A Deep Dive
The reaction between sodium carbonate (Na₂CO₃) and hydrochloric acid (HCl) is a classic example of an acid-base reaction, specifically a neutralization reaction. Understanding this reaction, including its balanced equation and the stoichiometry involved, is crucial for various applications in chemistry, from titrations to understanding industrial processes. This comprehensive article delves into the intricacies of this reaction, exploring the balanced equation, the steps involved in balancing it, the different products formed, and the practical implications of this seemingly simple chemical process.
Understanding the Reactants: Sodium Carbonate and Hydrochloric Acid
Before diving into the balanced equation, let's briefly review the properties of the reactants:
Sodium Carbonate (Na₂CO₃): Also known as washing soda or soda ash, sodium carbonate is a white, crystalline powder. It's a strong base, readily dissolving in water to form a moderately alkaline solution. Its alkaline nature makes it useful in many applications, including water softening, cleaning agents, and in the manufacturing of glass and soap.
Hydrochloric Acid (HCl): A strong, corrosive acid, hydrochloric acid is a colorless solution of hydrogen chloride (HCl) in water. It's a common laboratory reagent used extensively in various industrial processes, including metal cleaning, leather processing, and food production. Its high acidity makes it crucial for many chemical reactions.
The Balanced Equation: A Step-by-Step Derivation
The reaction between sodium carbonate and hydrochloric acid produces sodium chloride, water, and carbon dioxide. The unbalanced equation is:
Na₂CO₃(aq) + HCl(aq) → NaCl(aq) + H₂O(l) + CO₂(g)
Notice that this equation is not balanced. To balance it, we need to ensure that the number of atoms of each element is the same on both sides of the equation. Here's a step-by-step approach:
- Balance the Sodium (Na) atoms: There are two sodium atoms on the left (in Na₂CO₃) and one on the right (in NaCl). To balance this, we add a coefficient of 2 in front of NaCl:
Na₂CO₃(aq) + HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g)
- Balance the Chlorine (Cl) atoms: Now, we have two chlorine atoms on the right (in 2NaCl) and only one on the left (in HCl). We add a coefficient of 2 in front of HCl:
Na₂CO₃(aq) + 2HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g)
-
Balance the Hydrogen (H) atoms: There are two hydrogen atoms on both sides of the equation (in 2HCl and in 2H in H₂O). These are already balanced.
-
Balance the Carbon (C) atoms: There's one carbon atom on both sides (in Na₂CO₃ and CO₂). This is balanced.
-
Balance the Oxygen (O) atoms: There are three oxygen atoms on the left (in Na₂CO₃) and three on the right (in H₂O and CO₂). This is also balanced.
Therefore, the fully balanced equation is:
Na₂CO₃(aq) + 2HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g)
Understanding the Products: Sodium Chloride, Water, and Carbon Dioxide
The reaction produces three distinct products:
-
Sodium Chloride (NaCl): Common table salt, sodium chloride is a highly soluble ionic compound. It remains dissolved in the aqueous solution.
-
Water (H₂O): Water is formed as a byproduct of the neutralization reaction between the carbonate ion (CO₃²⁻) and the hydrogen ions (H⁺) from the hydrochloric acid.
-
Carbon Dioxide (CO₂): A gaseous product, carbon dioxide is released as bubbles during the reaction. This is easily observable as effervescence.
Stoichiometry and Mole Ratios
The balanced equation provides crucial information about the stoichiometry of the reaction. The coefficients in the balanced equation represent the mole ratios of the reactants and products. For example:
- 1 mole of Na₂CO₃ reacts with 2 moles of HCl.
- 1 mole of Na₂CO₃ produces 2 moles of NaCl, 1 mole of H₂O, and 1 mole of CO₂.
This stoichiometric information is essential for performing quantitative analysis, such as titrations, where the concentration of one reactant can be determined by reacting it with a known quantity of the other reactant.
Practical Applications and Importance
The reaction between sodium carbonate and hydrochloric acid has several practical applications:
-
Titration: This reaction is frequently used in acid-base titrations to determine the concentration of either sodium carbonate or hydrochloric acid solutions. The effervescence of CO₂ provides a visual endpoint indicator in some titrations.
-
Chemical Analysis: The reaction can be utilized in qualitative and quantitative chemical analysis for the determination of carbonate ions or acid concentration.
-
Industrial Processes: The reaction plays a role in various industrial processes involving the neutralization of acidic or alkaline waste streams.
-
Understanding Buffer Systems: This reaction illustrates the interaction between a weak base (carbonate ion) and a strong acid, providing insights into buffer systems and pH control.
Further Considerations: Reaction Rate and Factors Affecting It
The rate of the reaction between sodium carbonate and hydrochloric acid can be influenced by several factors:
-
Concentration of Reactants: Higher concentrations of reactants generally lead to a faster reaction rate.
-
Temperature: Increasing the temperature usually accelerates the reaction rate, as it increases the kinetic energy of the reacting molecules.
-
Surface Area: In the case of solid sodium carbonate reacting with liquid hydrochloric acid, increasing the surface area of the solid (e.g., by using finely powdered sodium carbonate) can increase the reaction rate.
-
Presence of Catalysts: Although not commonly used, certain catalysts could theoretically alter the reaction rate.
Conclusion: A Fundamental Reaction with Broad Applications
The reaction between sodium carbonate and hydrochloric acid, represented by the balanced equation Na₂CO₃(aq) + 2HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g), is a fundamental chemical reaction with broad implications across various fields. Understanding the balanced equation, the stoichiometry involved, and the factors influencing the reaction rate is crucial for anyone studying chemistry, performing chemical analyses, or working in industries where such reactions occur. The seemingly simple equation unlocks a deeper understanding of acid-base chemistry and its practical applications in the world around us. This reaction serves as a cornerstone for numerous further chemical explorations and applications. Its study provides a solid foundation for more complex chemical phenomena and processes.
Latest Posts
Latest Posts
-
In The Hershey And Chase Experiment Radioactively Labeled
Mar 17, 2025
-
Write 98 As A Product Of Prime Factors
Mar 17, 2025
-
English Scientist Who Coined The Term Cell Crossword
Mar 17, 2025
-
Examples Of Push And Pull Forces
Mar 17, 2025
-
The Final Products Of Glycolysis Are
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Sodium Carbonate And Hydrochloric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
