How Do You Get Number Of Neutrons
Juapaving
Mar 07, 2025 · 5 min read
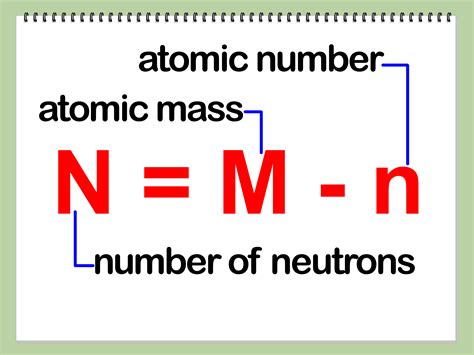
Table of Contents
How Do You Get the Number of Neutrons? A Comprehensive Guide
Determining the number of neutrons in an atom is a fundamental concept in chemistry and physics. Understanding this allows us to delve into the properties of elements, isotopes, and nuclear reactions. This comprehensive guide will explore various methods and concepts related to calculating the number of neutrons, moving beyond simple memorization towards a deeper understanding of atomic structure.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the methods of calculating neutron numbers, let's establish a solid foundation in atomic structure. An atom consists of three fundamental subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number (Z).
- Neutrons: Neutrally charged particles also residing in the nucleus. Neutrons contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons usually equals the number of protons in a neutral atom.
The mass number (A) of an atom represents the total number of protons and neutrons in its nucleus. This is crucial for neutron calculations.
Calculating the Number of Neutrons: The Fundamental Formula
The most straightforward way to determine the number of neutrons (N) in an atom is using the following formula:
N = A - Z
Where:
- N is the number of neutrons
- A is the mass number (total number of protons and neutrons)
- Z is the atomic number (number of protons)
This formula highlights the relationship between the mass number, atomic number, and the number of neutrons. Knowing any two of these values allows you to calculate the third.
Obtaining the Mass Number (A) and Atomic Number (Z): Essential Information
To use the formula effectively, you need to know the mass number (A) and atomic number (Z) of the atom in question. These values are readily available from various sources:
1. Periodic Table: Your First Stop
The periodic table is the chemist's best friend! Each element's square contains its atomic number (Z), usually displayed as a whole number above the element symbol. While the periodic table usually doesn't directly show the mass number, it provides the standard atomic weight, which is an average of the mass numbers of naturally occurring isotopes.
2. Isotopic Notation: Specifying Specific Isotopes
Many elements exist as isotopes – atoms of the same element with varying numbers of neutrons. Isotopic notation clarifies this difference:
<sup>A</sup><sub>Z</sub>X
Where:
- X is the element symbol (e.g., C for carbon, O for oxygen)
- Z is the atomic number
- A is the mass number
This notation directly provides both A and Z, making neutron calculation simple. For example, <sup>12</sup><sub>6</sub>C (carbon-12) has A = 12 and Z = 6.
3. Mass Spectrometry: Determining Isotopic Abundances
Mass spectrometry is a powerful analytical technique that precisely measures the mass-to-charge ratio of ions. It's particularly useful for determining the abundance of different isotopes within a sample. By analyzing the mass spectrum, scientists can identify the mass number of each isotope and its relative abundance.
Examples of Neutron Calculation
Let's work through some examples to illustrate the process:
Example 1: Carbon-12 (<sup>12</sup><sub>6</sub>C)
- A (mass number) = 12
- Z (atomic number) = 6
N = A - Z = 12 - 6 = 6 neutrons
Example 2: Uranium-235 (<sup>235</sup><sub>92</sub>U)
- A (mass number) = 235
- Z (atomic number) = 92
N = A - Z = 235 - 92 = 143 neutrons
Example 3: Determining the number of neutrons in Oxygen-18 given its atomic number is 8.
- A (mass number) = 18 (from the isotope name)
- Z (atomic number) = 8
N = A - Z = 18 - 8 = 10 neutrons
Beyond the Basics: Isotopes and Isotopic Abundance
The concept of isotopes is critical in understanding neutron numbers. Isotopes of the same element share the same number of protons but differ in their neutron count. This variation affects their mass and sometimes their radioactive properties.
For instance, carbon has three naturally occurring isotopes: carbon-12 (<sup>12</sup>C), carbon-13 (<sup>13</sup>C), and carbon-14 (<sup>14</sup>C). Each isotope has a different number of neutrons: 6, 7, and 8, respectively.
The isotopic abundance refers to the relative proportion of each isotope in a naturally occurring sample of an element. This is essential when considering the average atomic mass listed on the periodic table. The average atomic mass is a weighted average of the masses of all naturally occurring isotopes, reflecting their respective abundances.
Applications of Neutron Number Determination
The ability to calculate the number of neutrons has vast applications across various scientific fields:
- Nuclear Physics: Understanding neutron numbers is crucial for studying nuclear reactions, nuclear stability, and radioactive decay.
- Nuclear Medicine: Radioactive isotopes, often with specific neutron counts, are used in medical imaging and treatments.
- Material Science: The neutron count impacts material properties, influencing things like strength, conductivity, and reactivity.
- Geochemistry: Isotope ratios (and hence neutron numbers) are used to date geological samples and trace the origin of materials.
Advanced Concepts: Nuclear Binding Energy and Stability
The number of neutrons significantly influences nuclear stability. The strong nuclear force binds protons and neutrons together in the nucleus. However, the repulsive electrostatic force between protons competes with this binding force. The optimal neutron-to-proton ratio for stability varies with atomic number. Elements with too many or too few neutrons relative to protons tend to be radioactive and undergo decay to reach a more stable configuration. This stability is closely related to the nuclear binding energy, a measure of the energy required to disassemble a nucleus into its individual protons and neutrons.
Conclusion
Determining the number of neutrons in an atom is a fundamental skill in chemistry and physics. Using the simple formula N = A - Z, coupled with knowledge of the mass number and atomic number, allows for accurate calculations. Understanding isotopes and isotopic abundance adds depth to this knowledge, highlighting the variety within elements and the impact of neutron numbers on nuclear properties and applications. From basic atomic structure to advanced concepts like nuclear binding energy and stability, the ability to calculate neutron counts is a cornerstone of many scientific disciplines.
Latest Posts
Latest Posts
-
What Is The Prime Factorization 44
Mar 09, 2025
-
Moment Of Inertia Of Rectangular Plate
Mar 09, 2025
-
What Is The Function Of The Base Of The Microscope
Mar 09, 2025
-
How Many Sides Do A Octagon Have
Mar 09, 2025
-
How Many Valence Electrons In Br
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How Do You Get Number Of Neutrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
