How Do Compounds Differ From Elements
Juapaving
Mar 16, 2025 · 6 min read
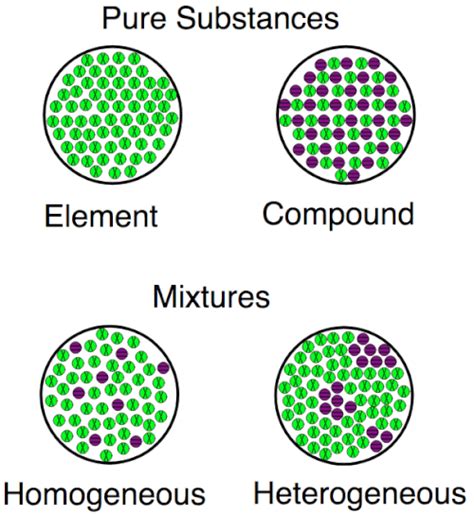
Table of Contents
How Do Compounds Differ From Elements? A Deep Dive into the Building Blocks of Matter
Understanding the fundamental building blocks of matter—elements and compounds—is crucial for grasping the intricacies of chemistry and the physical world around us. While seemingly simple, the distinction between elements and compounds reveals a fascinating complexity that governs the properties and behaviors of all substances. This comprehensive guide will explore the core differences between elements and compounds, delve into their characteristics, and provide examples to illuminate these fundamental concepts.
Defining Elements: The Foundation of Matter
An element is a pure substance consisting entirely of one type of atom. Atoms are the smallest units of matter that retain the chemical properties of an element. Each element is defined by the number of protons in its atomic nucleus, known as its atomic number. This number uniquely identifies the element and dictates its position on the periodic table, the organized chart of all known elements.
Key Characteristics of Elements:
-
Pure Substance: Elements are inherently pure, meaning they are not a mixture of other substances. They exist as individual atoms or, in some cases, as molecules composed of identical atoms (e.g., O<sub>2</sub>, oxygen gas).
-
Unique Atomic Number: Each element possesses a unique atomic number, which determines its chemical identity and properties. This number is unchangeable; you can't transform one element into another through ordinary chemical means.
-
Cannot be Broken Down: Elements are considered the simplest form of matter. They cannot be broken down into simpler substances through chemical reactions. Nuclear reactions, however, can alter the composition of an element's nucleus.
-
Distinct Properties: Elements exhibit distinct physical and chemical properties, such as melting point, boiling point, reactivity, and density. These properties are determined by the arrangement of electrons in the atom's electron shell and the strength of its nuclear forces.
-
Examples: Examples of elements include oxygen (O), hydrogen (H), carbon (C), gold (Au), iron (Fe), and chlorine (Cl). These elements, alone or in combination with other elements, make up everything around us.
Defining Compounds: The Marriage of Elements
A compound is a pure substance formed when two or more different elements combine chemically in a fixed ratio. This combination involves the formation of chemical bonds, strong forces that hold the atoms together. Unlike mixtures, compounds have a consistent composition and cannot be separated into their constituent elements through physical methods (like filtration or distillation).
Key Characteristics of Compounds:
-
Chemical Combination: Compounds are formed through chemical reactions, where atoms of different elements interact and bond together. This bonding involves the sharing or transfer of electrons.
-
Fixed Ratio: The elements within a compound are always present in a specific, fixed ratio. For example, water (H<sub>2</sub>O) always contains two hydrogen atoms for every one oxygen atom. This ratio is defined by the compound's chemical formula.
-
Distinct Properties from Constituent Elements: The properties of a compound are often very different from the properties of the elements that compose it. For instance, sodium (Na) is a highly reactive metal, and chlorine (Cl) is a toxic gas. However, their combination forms sodium chloride (NaCl), or table salt, a relatively inert and edible compound.
-
Can be Broken Down: Unlike elements, compounds can be broken down into their constituent elements through chemical reactions. This often requires supplying energy, such as through heating or electrolysis.
-
Examples: Examples of compounds include water (H<sub>2</sub>O), carbon dioxide (CO<sub>2</sub>), table salt (NaCl), glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>), and sulfuric acid (H<sub>2</sub>SO<sub>4</sub>). These compounds are vital for life and industrial processes.
The Crucial Differences: A Comparative Analysis
The following table summarizes the key differences between elements and compounds:
| Feature | Element | Compound |
|---|---|---|
| Definition | A pure substance consisting of only one type of atom. | A pure substance formed from two or more different elements chemically combined in a fixed ratio. |
| Composition | One type of atom | Two or more types of atoms |
| Chemical Bonds | No chemical bonds between different atoms (only within the atom itself) | Chemical bonds hold atoms together |
| Separation | Cannot be broken down chemically | Can be broken down chemically into its constituent elements |
| Properties | Unique properties determined by atomic number | Properties differ significantly from constituent elements |
| Ratio of Atoms | No ratio, it's a pure substance. | Fixed ratio of atoms in its chemical formula |
Exploring the World of Mixtures: A Third Category
It's essential to distinguish both elements and compounds from mixtures. Mixtures are physical combinations of two or more substances, where the substances retain their individual properties and can be separated by physical means. Unlike compounds, mixtures do not involve chemical bonding and do not have a fixed composition. For example, saltwater is a mixture of salt (NaCl, a compound) and water (H<sub>2</sub>O, a compound). The salt can be separated from the water through evaporation.
Beyond the Basics: Isotopes and Allotropes
The world of elements and compounds gets even more nuanced when we consider isotopes and allotropes.
Isotopes: Variations on an Element
Isotopes are atoms of the same element that have the same atomic number but different numbers of neutrons. This difference in neutron count results in variations in atomic mass. For example, carbon-12 (<sup>12</sup>C) and carbon-14 (<sup>14</sup>C) are isotopes of carbon; they both have six protons but have six and eight neutrons, respectively. While isotopes of the same element have similar chemical properties, they may differ in their physical properties, such as radioactivity (as seen with carbon-14, a radioactive isotope).
Allotropes: Different Forms of the Same Element
Allotropes are different structural modifications of the same element in the same physical state. These different forms arise from the various ways the atoms of the element can bond together. A classic example is carbon, which exists as diamond, graphite, and fullerenes (like buckminsterfullerene, or "buckyballs"). These allotropes differ significantly in their properties; diamond is extremely hard and transparent, graphite is soft and conductive, and fullerenes have unique molecular structures and properties.
The Importance of Understanding Elements and Compounds
The distinction between elements and compounds is fundamental to our understanding of chemistry and the physical world. This knowledge is crucial in various fields:
-
Material Science: Understanding the properties of elements and compounds allows scientists to design and synthesize new materials with specific desired characteristics.
-
Medicine: Knowledge of chemical reactions involving elements and compounds is critical for developing new drugs and therapies.
-
Environmental Science: Understanding the behavior of elements and compounds in the environment is vital for addressing environmental challenges, such as pollution control and climate change.
-
Food Science: The chemical composition of food, involving various elements and compounds, is essential for ensuring food safety and nutritional value.
Conclusion: A Foundation for Future Exploration
The difference between elements and compounds represents a cornerstone concept in chemistry and provides a framework for understanding the vast diversity of substances in the universe. By understanding these fundamental differences, we unlock the ability to explore the intricate relationships between matter and its properties, paving the way for innovation and advancements in various scientific and technological fields. From the simplest atoms to the most complex molecules, the principles governing elements and compounds are essential for comprehending the world around us. Further exploration into chemical bonding, stoichiometry, and chemical reactions will only deepen our appreciation of these crucial building blocks of matter.
Latest Posts
Latest Posts
-
Square Root Of 125 In Simplest Radical Form
Mar 16, 2025
-
Is Melting Of Wax A Physical Or Chemical Change
Mar 16, 2025
-
What Are Rows On The Periodic Table Called
Mar 16, 2025
-
Is Carbon Tetrachloride Ionic Or Covalent
Mar 16, 2025
-
Write The Formula For Sulfurous Acid
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How Do Compounds Differ From Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
