Is Carbon Tetrachloride Ionic Or Covalent
Juapaving
Mar 16, 2025 · 5 min read
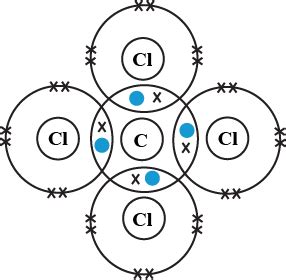
Table of Contents
Is Carbon Tetrachloride Ionic or Covalent? A Deep Dive into Chemical Bonding
Carbon tetrachloride (CCl₄), a colorless liquid with a characteristic ethereal odor, is a fascinating molecule that often sparks discussions about its bonding nature. The question, "Is carbon tetrachloride ionic or covalent?" is a fundamental one in chemistry, and understanding the answer requires exploring the concepts of electronegativity, bond polarity, and molecular geometry. This comprehensive guide will delve into these aspects, providing a clear and detailed explanation of why carbon tetrachloride is considered a covalent compound.
Understanding Chemical Bonding: Ionic vs. Covalent
Before we dissect the specifics of carbon tetrachloride, let's establish a foundational understanding of the two primary types of chemical bonds: ionic and covalent.
Ionic Bonds: The Transfer of Electrons
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This occurs when one atom, typically a metal with low electronegativity, donates one or more electrons to another atom, usually a nonmetal with high electronegativity. The atom that loses electrons becomes a positively charged cation, while the atom that gains electrons becomes a negatively charged anion. The strong electrostatic force between these ions holds them together in a crystalline lattice structure. Classic examples include sodium chloride (NaCl) and magnesium oxide (MgO).
Covalent Bonds: The Sharing of Electrons
In contrast to ionic bonds, covalent bonds involve the sharing of electrons between two atoms. This sharing occurs when atoms have similar electronegativities, meaning they have a comparable tendency to attract electrons. By sharing electrons, atoms achieve a more stable electron configuration, often fulfilling the octet rule (having eight electrons in their valence shell). Covalent bonds can be further classified as nonpolar or polar based on the electronegativity difference between the bonded atoms.
Electronegativity: A Key Determinant of Bond Type
Electronegativity is a crucial concept in understanding the nature of chemical bonds. It's a measure of an atom's ability to attract electrons towards itself within a chemical bond. Elements with high electronegativity strongly attract electrons, while those with low electronegativity hold electrons less tightly. The difference in electronegativity between two atoms dictates the nature of the bond formed between them.
A large electronegativity difference (typically greater than 1.7 on the Pauling scale) generally indicates an ionic bond. A small electronegativity difference (typically less than 0.5) indicates a nonpolar covalent bond, where electrons are shared almost equally. A moderate electronegativity difference (between 0.5 and 1.7) results in a polar covalent bond, where electrons are shared unequally, creating a partial positive and a partial negative charge on the atoms involved.
Analyzing Carbon Tetrachloride (CCl₄)
Now, let's apply this knowledge to carbon tetrachloride (CCl₄).
Electronegativity Values: Carbon and Chlorine
The electronegativity of carbon (C) is approximately 2.55, while the electronegativity of chlorine (Cl) is approximately 3.16. The difference in electronegativity between carbon and chlorine is 3.16 - 2.55 = 0.61.
The Covalent Nature of C-Cl Bonds
This electronegativity difference of 0.61 falls within the range that suggests a polar covalent bond. Although there's a slight difference in electronegativity, it's not large enough to classify the C-Cl bonds as ionic. The electrons are shared between carbon and chlorine, albeit unequally. Chlorine, being more electronegative, attracts the shared electrons more strongly, resulting in a slightly negative charge (δ-) on the chlorine atoms and a slightly positive charge (δ+) on the carbon atom.
Molecular Geometry and Bond Dipoles
Carbon tetrachloride has a tetrahedral molecular geometry. This means that the four chlorine atoms are arranged symmetrically around the central carbon atom. While each individual C-Cl bond is polar (possessing a bond dipole), the symmetry of the molecule causes these bond dipoles to cancel each other out. Therefore, the overall molecule is nonpolar. This means that despite the polar nature of the individual bonds, the molecule as a whole does not have a net dipole moment.
Further Evidence for Covalent Bonding in CCl₄
Several other factors reinforce the covalent nature of carbon tetrachloride:
-
Low Melting and Boiling Points: Ionic compounds typically have high melting and boiling points due to the strong electrostatic forces between ions in the crystal lattice. Carbon tetrachloride, however, has relatively low melting and boiling points, consistent with a covalent compound with weaker intermolecular forces.
-
Poor Electrical Conductivity: Ionic compounds conduct electricity when molten or dissolved in water because the ions are free to move. Carbon tetrachloride is a poor conductor of electricity in all states, further supporting the covalent nature of its bonds.
-
Solubility: Ionic compounds tend to be soluble in polar solvents like water, while covalent compounds are often soluble in nonpolar solvents. Carbon tetrachloride is soluble in nonpolar solvents, providing additional evidence of its covalent nature.
-
Formation through Sharing, Not Transfer: The formation of carbon tetrachloride involves the sharing of electrons between carbon and chlorine atoms to achieve stable electron configurations, not a complete transfer of electrons like in ionic bond formation.
Addressing Common Misconceptions
Some students may mistakenly believe that the presence of any electronegativity difference automatically implies an ionic bond. However, this is inaccurate. The magnitude of the electronegativity difference is key. A small difference, as seen in carbon tetrachloride, results in a polar covalent bond, but the overall molecular symmetry can lead to a nonpolar molecule.
Another misconception involves confusing polarity of individual bonds with the polarity of the entire molecule. While the C-Cl bonds in CCl₄ are polar, the symmetrical arrangement cancels out their dipole moments, resulting in a nonpolar molecule.
Conclusion: Carbon Tetrachloride is Covalent
In conclusion, overwhelming evidence points to carbon tetrachloride (CCl₄) being a covalent compound. While the C-Cl bonds possess some degree of polarity due to the electronegativity difference between carbon and chlorine, the symmetrical tetrahedral geometry of the molecule leads to the cancellation of individual bond dipoles, resulting in an overall nonpolar molecule. Its physical and chemical properties, including low melting and boiling points, poor electrical conductivity, and solubility in nonpolar solvents, all further support its classification as a covalent compound. The sharing of electrons, rather than a complete transfer, in the bond formation process solidifies this conclusion. Understanding the nuances of electronegativity, bond polarity, and molecular geometry is crucial to accurately classifying chemical bonds and predicting the properties of compounds.
Latest Posts
Latest Posts
-
How Many Centimeters Are In 3 Inches
Mar 16, 2025
-
Examples Of Pulling And Pushing Forces
Mar 16, 2025
-
Refraction Causes The Bottom Of A Swimming Pool To Appear
Mar 16, 2025
-
Is Black Or Brown Hair Dominant
Mar 16, 2025
-
Which Of These Is An Example Of A Chemical Change
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Is Carbon Tetrachloride Ionic Or Covalent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
