Give The Temperature And Pressure At Stp
Juapaving
Mar 29, 2025 · 5 min read
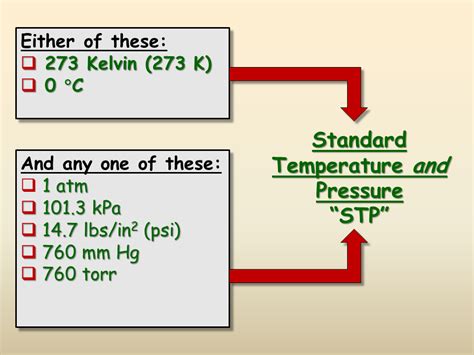
Table of Contents
Give the Temperature and Pressure at STP: A Comprehensive Guide
Standard Temperature and Pressure (STP) is a fundamental concept in chemistry and physics, providing a baseline for comparing and analyzing the behavior of gases and other substances. Understanding STP is crucial for various scientific calculations, experiments, and industrial applications. This comprehensive guide will delve deep into the definition of STP, its variations, the significance of specifying STP, practical applications, and common misconceptions. We will also explore the implications of using different STP definitions and their impact on experimental results.
What is Standard Temperature and Pressure (STP)?
STP refers to a set of standard conditions for temperature and pressure used primarily for comparing the properties of gases. It allows scientists worldwide to obtain consistent and comparable results, regardless of location or experimental setup. However, it's important to note that there isn't a single universally accepted definition of STP. Two primary definitions are commonly used:
1. The Older Definition:
- Temperature: 0° Celsius (273.15 Kelvin)
- Pressure: 1 atmosphere (atm) or 101.325 kilopascals (kPa) or 760 millimeters of mercury (mmHg)
This definition was widely used for many years and is still encountered in some textbooks and older scientific literature.
2. The IUPAC Definition (Recommended):
- Temperature: 273.15 Kelvin (0° Celsius)
- Pressure: 100 kilopascals (kPa)
The International Union of Pure and Applied Chemistry (IUPAC) recommends this definition, which is becoming increasingly prevalent in modern scientific work. The difference in pressure between the older and IUPAC definitions is significant and can affect calculations, especially those involving ideal gas law.
Why Specify Standard Temperature and Pressure?
The properties of gases, unlike solids and liquids, are highly sensitive to changes in temperature and pressure. Gas volume, density, and other characteristics vary significantly with even minor fluctuations in these parameters. Specifying STP provides a consistent reference point, enabling:
- Accurate Comparisons: Researchers can compare experimental results from different labs and different times accurately if the data is reported under the same standard conditions.
- Reproducible Results: Using STP ensures that experiments can be replicated consistently, leading to reliable scientific findings.
- Simplified Calculations: Many gas laws, such as the Ideal Gas Law (PV = nRT), require the use of standard conditions for precise calculations. Without STP, the results would vary depending on the ambient temperature and pressure.
- Industry Standardization: STP is essential for various industrial processes involving gases, such as the production and transportation of gases, chemical reactions, and quality control.
The Significance of the Pressure Difference between Definitions
The difference in pressure between the older (1 atm) and the IUPAC (100 kPa) definitions is approximately 1.325 kPa (or about 1%). While seemingly small, this difference can lead to noticeable discrepancies, particularly in precise scientific measurements. The volume of a gas, for example, will be slightly different under these two conditions. This necessitates careful attention to which STP definition is being employed when interpreting or comparing data from various sources.
Practical Applications of STP
The use of STP extends across many disciplines, including:
- Chemistry: STP is used in stoichiometric calculations involving gases, determining molar volumes, and understanding gas behavior in various chemical reactions.
- Physics: It's crucial for studying gas dynamics, thermodynamics, and other physical phenomena related to gases.
- Environmental Science: STP is used in analyzing air quality, monitoring pollutants, and studying atmospheric processes.
- Meteorology: While weather conditions rarely conform to STP, understanding STP helps in interpreting and extrapolating meteorological data.
- Engineering: Various engineering applications, particularly those involving gases (e.g., combustion engines, pipeline transport), use STP as a reference for calculations and designs.
Ideal Gas Law and STP
The Ideal Gas Law, PV = nRT, is a fundamental equation in chemistry that relates the pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R) of a gas. When working with the Ideal Gas Law, it is vital to specify the temperature and pressure used, and STP provides a convenient standard. The ideal gas constant (R) also has different values depending on the units used for pressure and volume; hence, attention to units is critical for accurate calculations.
Common Misconceptions about STP
Several misconceptions surrounding STP should be clarified:
- STP is universally defined: As discussed earlier, there are two main definitions of STP, and it's crucial to specify which one is being used.
- STP reflects real-world conditions: STP is a theoretical standard; rarely do real-world atmospheric conditions align perfectly with STP.
- STP is only for gases: While primarily used for gases, the concept of standard conditions can be extended to other substances, particularly liquids, to facilitate comparisons of properties.
Dealing with Non-STP Conditions
Often, experimental data are collected under conditions that differ from STP. To make comparisons with data collected at STP, or to use data in calculations requiring STP, it's necessary to correct the measurements using the appropriate gas laws. This often involves using the combined gas law or other relationships derived from the Ideal Gas Law. These corrections are essential for ensuring the accuracy and reliability of scientific findings.
Conclusion
Standard Temperature and Pressure (STP) is an indispensable concept in science and industry. While the lack of a single universally accepted definition can lead to some confusion, understanding the two primary definitions and their implications is crucial for accurate scientific work. Specifying which definition is used is vital for ensuring consistent and reproducible results. The importance of STP extends beyond simple calculations; it serves as a cornerstone for comparing experimental data, establishing industrial standards, and understanding the behavior of gases under controlled conditions. The meticulous application of STP ensures the precision and reliability of scientific endeavors across diverse disciplines. Remember to always explicitly state which definition of STP you are using in your work to avoid ambiguity and ensure clarity.
Latest Posts
Latest Posts
-
How To Calculate Tension In A Cable
Mar 31, 2025
-
Solve The Equation Round To The Nearest Hundredth
Mar 31, 2025
-
A Tool With A Curved Blade
Mar 31, 2025
-
In Situ And Ex Situ Conservation
Mar 31, 2025
-
As You Move Left To Right On The Periodic Table
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Give The Temperature And Pressure At Stp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
