As You Move Left To Right On The Periodic Table
Juapaving
Mar 31, 2025 · 7 min read
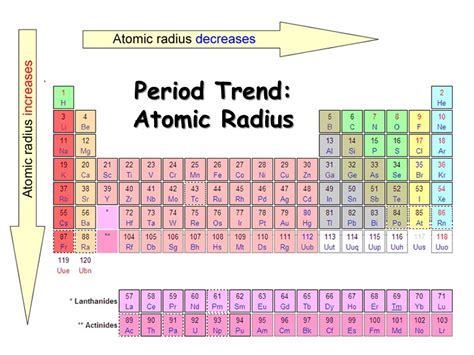
Table of Contents
As You Move Left to Right on the Periodic Table: Exploring Trends in Atomic Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the trends that emerge as you move across a period (left to right) is crucial for grasping the behavior of elements and predicting their reactivity. This comprehensive exploration delves into the fascinating changes in atomic radius, ionization energy, electron affinity, electronegativity, and metallic character as we traverse the periodic table from left to right.
Atomic Radius: A Shrinking Trend
Atomic radius, the distance from the nucleus to the outermost electron shell, exhibits a consistent decrease as you move from left to right across a period. This trend is primarily attributed to the increasing nuclear charge. As we progress across a period, the number of protons in the nucleus increases, leading to a stronger positive charge. This stronger positive charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius. The added electrons are also filling the same principal energy level (shell), not adding a new energy level, which further contributes to the reduced atomic size.
Why is this decrease significant? The atomic radius directly impacts an element's reactivity and the types of chemical bonds it can form. Smaller atoms tend to be more reactive as their valence electrons are held more tightly and are less likely to be involved in bonding.
Ionization Energy: The Increasing Cost of Removing Electrons
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. As we traverse a period from left to right, ionization energy generally increases. This is a direct consequence of the increasing nuclear charge. The stronger attraction between the nucleus and electrons makes it increasingly difficult to remove an electron. The added electrons are also experiencing greater shielding effects from inner-shell electrons, meaning that the effective nuclear charge increases as we move across the period, making it harder to remove an electron.
First Ionization Energy vs. Subsequent Ionization Energies: It's important to distinguish between the first ionization energy (removing the first electron) and subsequent ionization energies (removing subsequent electrons). Each successive ionization energy is significantly higher than the previous one because removing an electron leaves a more positively charged ion, making it even harder to remove another electron.
Exceptions to the Trend: While the general trend is upward, there are minor irregularities. These variations often arise from the stability associated with half-filled or completely filled subshells. For instance, removing an electron from a half-filled subshell requires more energy than removing one from a partially filled subshell.
Electron Affinity: The Attraction to Extra Electrons
Electron affinity refers to the energy change when an electron is added to a neutral gaseous atom. While not as consistently predictable as ionization energy, electron affinity generally increases across a period. As we move rightward, the increased nuclear charge enhances the attraction for an additional electron, leading to a more negative electron affinity value (meaning energy is released). The smaller atomic size also contributes to this increased attraction.
Exceptions and Irregularities: However, there are exceptions to this trend. For instance, the electron affinity of some elements, notably the noble gases, is close to zero or even positive, indicating they have little or no tendency to accept an additional electron. This is because their valence electron shells are already full and adding another electron would require it to be placed into a higher energy level, making it energetically unfavorable.
Electronegativity: Tug-of-War for Electrons
Electronegativity describes an atom's ability to attract electrons within a chemical bond. It also generally increases across a period. The increasing nuclear charge as we move from left to right leads to a stronger pull on shared electrons in a covalent bond, resulting in higher electronegativity.
Pauling Scale: Electronegativity values are usually expressed using the Pauling scale, where fluorine (the most electronegative element) is assigned a value of 4.0.
Impact on Bonding: Electronegativity differences between atoms influence the nature of the chemical bond formed. A large electronegativity difference leads to ionic bonding (transfer of electrons), while a small difference results in covalent bonding (sharing of electrons).
Metallic Character: A Gradual Transition
Metallic character refers to the properties typically associated with metals, such as conductivity, malleability, and ductility. Metallic character generally decreases as you move from left to right across a period. This is due to the increasing effective nuclear charge, which pulls the valence electrons more tightly to the nucleus. This tighter hold on valence electrons reduces their ability to move freely, diminishing the metallic properties.
Left Side vs. Right Side: Elements on the left side of the periodic table are typically metals, characterized by their ability to lose electrons easily and form positive ions. In contrast, elements on the right side are nonmetals, which tend to gain electrons to form negative ions.
Oxidation States: Reflecting Electron Configuration
The oxidation state of an element indicates the number of electrons it has gained or lost during chemical bonding. As we move across a period, the common oxidation states generally change. Elements on the left side have low positive oxidation states because they easily lose electrons. As you move towards the right, the oxidation states become increasingly negative, signifying a greater tendency to gain electrons.
Predicting Oxidation States: The electron configuration of an element helps in predicting its common oxidation states. Elements often form stable ions by gaining or losing electrons to achieve a noble gas electron configuration.
Summary of Trends Across a Period
| Property | Trend Across a Period (Left to Right) | Reason |
|---|---|---|
| Atomic Radius | Decreases | Increasing nuclear charge |
| Ionization Energy | Increases | Increasing nuclear charge, ineffective shielding |
| Electron Affinity | Generally increases | Increasing nuclear charge |
| Electronegativity | Increases | Increasing nuclear charge |
| Metallic Character | Decreases | Increasing nuclear charge, tighter hold on valence electrons |
| Oxidation States | Shifts from positive to negative | Changes in electron configuration and tendency to gain or lose electrons |
Exceptions and Irregularities: Why the Simple Rules Aren't Always Simple
While the general trends discussed above provide a useful framework for understanding the periodic table, it's essential to acknowledge that exceptions and irregularities exist. These deviations are often attributable to:
- Electron-electron repulsions: Increased electron-electron repulsions within a subshell can affect the effective nuclear charge and influence atomic properties.
- Shielding effects: The extent of shielding of the valence electrons by inner-shell electrons is not uniform across the period.
- Subshell stability: Half-filled and completely filled subshells possess added stability, leading to slight variations in ionization energies and electron affinities.
- Relativistic effects: At higher atomic numbers, relativistic effects (due to the high speeds of inner electrons) can influence atomic properties, particularly atomic radius.
Understanding these exceptions requires a deeper understanding of quantum mechanics and the intricacies of electron configurations.
Applications of Periodic Trends
The trends in atomic properties discussed above are not mere academic exercises. They have wide-ranging applications in various fields, including:
- Predicting chemical reactivity: Knowing the electronegativity and ionization energy helps predict the type of chemical bonds an element will form and its reactivity with other elements.
- Material science: Understanding the relationship between atomic properties and metallic character is crucial in designing new materials with specific properties.
- Catalysis: The ability of elements to gain or lose electrons, as reflected in their oxidation states, plays a vital role in their catalytic activity.
- Biological systems: The properties of elements significantly influence their roles in biological systems, such as the role of transition metals in enzymes.
Conclusion
Understanding the trends across a period on the periodic table is fundamental to understanding chemical behavior. While general trends exist for atomic radius, ionization energy, electron affinity, electronegativity, and metallic character, exceptions and irregularities highlight the complexities of atomic interactions. This knowledge forms the basis for predicting chemical reactions, designing new materials, and understanding the roles of elements in biological systems. By appreciating both the overarching trends and the nuances of exceptions, we can develop a more complete and insightful understanding of the fascinating world of chemistry. Further exploration into the specific electronic configurations and quantum mechanical principles underlying these trends will enhance your understanding of the periodic table and the elements it encapsulates.
Latest Posts
Latest Posts
-
Air Moves From High To Low Pressure
Apr 02, 2025
-
The Smallest Particle Of An Element Is A N
Apr 02, 2025
-
How Many Neutrons Does A Hydrogen Atom Have
Apr 02, 2025
-
What Is Nickel Used For In Everyday Life
Apr 02, 2025
-
A Tetrad Is Made Up Of
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about As You Move Left To Right On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
