Freezing Point Of Water In Kelvin
Juapaving
Mar 15, 2025 · 5 min read
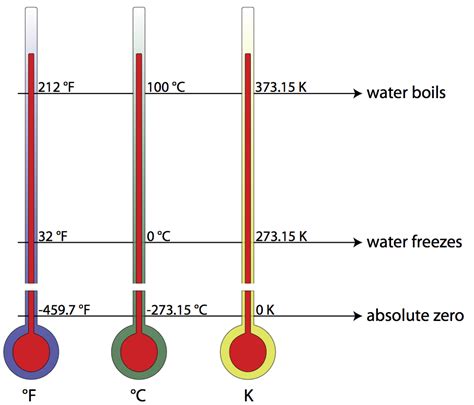
Table of Contents
Freezing Point of Water in Kelvin: A Deep Dive
The freezing point of water is a fundamental constant in science, serving as a crucial reference point across various disciplines. While commonly known as 0 degrees Celsius or 32 degrees Fahrenheit, its expression in Kelvin is equally important, particularly within scientific and engineering contexts. This article delves into the intricacies of water's freezing point in Kelvin, exploring its significance, underlying principles, and applications.
Understanding the Kelvin Scale
Before delving into the freezing point of water specifically, it's crucial to understand the Kelvin scale itself. Unlike Celsius and Fahrenheit, which are relative scales based on arbitrary reference points (the freezing and boiling points of water), the Kelvin scale is an absolute thermodynamic temperature scale. This means it starts at absolute zero – the theoretical point where all molecular motion ceases.
Key characteristics of the Kelvin scale:
- Absolute Zero: 0 Kelvin (0 K) represents absolute zero, the lowest possible temperature.
- No Negative Values: The Kelvin scale doesn't employ negative values, simplifying many calculations in thermodynamics and physics.
- Relationship to Celsius: The Kelvin scale is directly related to the Celsius scale: K = °C + 273.15. This means a temperature difference of 1 Kelvin is equal to a temperature difference of 1 degree Celsius.
The Freezing Point of Water in Kelvin: 273.15 K
The freezing point of water at standard atmospheric pressure (101.325 kPa) is precisely 273.15 Kelvin (K). This value is derived from the relationship between the Kelvin and Celsius scales. Since 0°C is the freezing point of water on the Celsius scale, adding 273.15 gives us the equivalent temperature in Kelvin.
Why is precision important?
The use of 273.15 K, rather than simply 273 K, reflects the high level of accuracy required in scientific measurements. The ".15" accounts for subtle variations and ensures consistency across different experiments and calculations. This seemingly small difference can significantly impact highly sensitive scientific applications.
Factors Affecting the Freezing Point of Water
While 273.15 K is the standard freezing point, several factors can influence the precise temperature at which water freezes:
1. Pressure:
Pressure significantly affects the freezing point of water. At higher pressures, the freezing point decreases slightly. This unusual property, where the solid phase is less dense than the liquid phase, is unique to water and has profound implications for various natural phenomena like glacial movement.
2. Impurities:
Dissolved substances, like salts and other solutes, lower the freezing point of water. This is the principle behind using salt to de-ice roads in winter. The presence of impurities disrupts the water molecules' ability to form a crystalline structure, requiring a lower temperature to initiate freezing. This phenomenon, known as freezing point depression, is directly proportional to the concentration of the dissolved substance.
3. Supercooling:
Under specific conditions, water can be cooled below its freezing point without actually freezing. This phenomenon, known as supercooling, occurs when there are few nucleation sites for ice crystal formation. Supercooled water is metastable and will quickly freeze upon introduction of an ice crystal or other disturbance.
4. Isotopic Composition:
The isotopic composition of water molecules can also slightly affect the freezing point. Water molecules containing heavier isotopes of hydrogen (deuterium) and oxygen have slightly higher freezing points.
Significance of the Freezing Point of Water in Kelvin
The freezing point of water in Kelvin holds immense significance across various scientific and engineering fields:
1. Thermodynamics:
Kelvin temperature is crucial for thermodynamic calculations, as many equations rely on absolute temperature. Understanding the freezing point in Kelvin is vital for determining enthalpy changes, entropy changes, and equilibrium constants in various water-related processes.
2. Cryogenics:
Cryogenics, the study of very low temperatures, relies heavily on the Kelvin scale. Precise knowledge of water's freezing point in Kelvin is critical in designing and operating cryogenic systems.
3. Materials Science:
Many material properties change with temperature. Knowing the freezing point of water in Kelvin is essential in designing and testing materials that will be exposed to freezing temperatures. This is particularly important in fields such as construction and aerospace engineering.
4. Meteorology and Climatology:
Accurate measurement and understanding of temperature in Kelvin are crucial for meteorological and climatological studies. The freezing point of water serves as a benchmark in understanding various weather phenomena and climate models.
5. Chemistry and Biochemistry:
Many chemical and biochemical reactions are temperature-dependent. Using the Kelvin scale allows for precise calculations of reaction rates and equilibrium constants, especially in processes involving water.
Applications of the Freezing Point of Water in Kelvin
The precise knowledge of water's freezing point in Kelvin finds practical application in various domains:
- Food preservation: The freezing point is critical in food preservation techniques, determining the effective temperature for freezing and preventing spoilage.
- Water treatment: Understanding the freezing point is vital in water purification and treatment processes.
- Environmental monitoring: Measuring temperature in Kelvin is essential for monitoring environmental conditions and assessing the impact of climate change.
- Medical applications: Maintaining precise temperatures around the freezing point of water is critical in various medical applications, including cryosurgery and organ preservation.
- Industrial processes: Many industrial processes, especially those involving water, rely on precise temperature control based on the Kelvin scale.
Beyond the Standard: Deviations and Anomalies
While 273.15 K represents the standard freezing point, it's essential to acknowledge that this is under idealized conditions. Real-world scenarios often involve deviations from this value due to the factors mentioned previously (pressure, impurities, etc.). Scientists and engineers must account for these variations depending on the specific application. For instance, calculating the freezing point of seawater requires considering the salinity-induced freezing point depression.
Conclusion
The freezing point of water in Kelvin, precisely 273.15 K, isn't just a number; it's a cornerstone of scientific understanding. Its precise measurement and its implications for various fields underscore its importance. From thermodynamic calculations to cryogenic engineering, understanding this fundamental constant is crucial for accurate measurements, efficient designs, and a deeper grasp of the world around us. The intricate relationship between temperature, pressure, and impurities, as it relates to water's freezing point, highlights the complexity and nuance inherent in seemingly simple phenomena. Continued research and exploration in these areas will undoubtedly yield further insights into the behavior of water and its significance for various scientific disciplines. This continuous refinement of our understanding of such fundamental concepts allows for advancements in various technological and scientific fields, impacting our daily lives in profound ways.
Latest Posts
Latest Posts
-
Whats The Square Root Of 4
Mar 15, 2025
-
What Is The Unit Of Acceleration
Mar 15, 2025
-
1 X 2 1 X 3
Mar 15, 2025
-
Melting Point Of Water In Celsius
Mar 15, 2025
-
How Tall Is 63 Inches In Feet
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point Of Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
