Melting Point Of Water In Celsius
Juapaving
Mar 15, 2025 · 5 min read
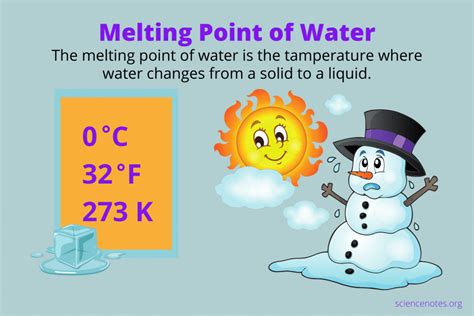
Table of Contents
The Melting Point of Water in Celsius: A Deep Dive
The melting point of water, a seemingly simple concept, is actually a fascinating area of study with implications across numerous scientific disciplines. Understanding this seemingly straightforward property unlocks a deeper understanding of the unique characteristics of water and its crucial role in our planet's ecosystems and countless industrial processes. This article delves into the intricacies of water's melting point in Celsius, exploring its definition, influencing factors, anomalies, and practical applications.
Defining the Melting Point of Water
The melting point of a substance is the temperature at which it transitions from a solid state (ice, in the case of water) to a liquid state (water). For water, under standard atmospheric pressure (1 atmosphere or 101.325 kPa), this transition occurs at 0° Celsius (0°C). This seemingly simple statement, however, belies a wealth of complex interactions at the molecular level.
The Role of Hydrogen Bonds
Water's unique melting point is directly related to its molecular structure and the strong hydrogen bonds that exist between water molecules (H₂O). These hydrogen bonds are relatively strong intermolecular forces that hold the water molecules together in a rigid crystalline structure in ice. To melt the ice, sufficient energy must be supplied to overcome these bonds and allow the molecules to move more freely, transitioning into the liquid phase.
Pressure's Influence on the Melting Point
While 0°C is the standard melting point, it's crucial to understand that the melting point of water is pressure-dependent. This is unusual, as for most substances, increasing pressure increases the melting point. However, water exhibits a unique negative slope in its phase diagram. This means that increasing pressure on ice actually lowers its melting point. This is because the liquid phase of water is denser than its solid phase (ice), which is an anomaly found in very few substances. The increased pressure favors the denser liquid phase, leading to melting at a lower temperature.
This phenomenon is observable in ice skating. The pressure exerted by the skate blades on the ice lowers the melting point locally, creating a thin layer of liquid water that reduces friction and allows for smoother gliding.
Factors Affecting the Melting Point of Water
Several factors, beyond pressure, can subtly influence the melting point of water:
Impurities and Dissolved Substances
The presence of impurities or dissolved substances in water can alter its melting point. Generally, dissolved substances depress the freezing point (and consequently, the melting point). This phenomenon is known as freezing-point depression and is a colligative property, meaning it depends on the concentration of solute particles, not their identity. This principle is utilized in applications like de-icing roads where salt is added to lower the freezing point of water, preventing ice formation at temperatures slightly below 0°C.
Isotopic Composition
The isotopic composition of water also plays a minor role. Water molecules can contain different isotopes of hydrogen (protium, deuterium, and tritium) and oxygen (¹⁶O, ¹⁷O, and ¹⁸O). Heavier isotopes, like deuterium (²H or D), form heavier water molecules (D₂O, also known as heavy water), which have higher melting points than regular water (H₂O). This difference, however, is relatively small, typically around 3.8°C.
Environmental Conditions
While less significant than the factors discussed above, environmental conditions such as the presence of air bubbles or other contaminants within the ice sample can also have a minor effect on the observed melting point. These factors might slightly alter the heat transfer processes during melting and affect the precise temperature measurement.
Anomalies and Unique Properties of Water
Water's behavior around its melting point highlights several anomalous properties that make it unique:
Density Anomaly
The most significant anomaly is the lower density of ice compared to liquid water. This is unusual; most substances are denser in their solid state than in their liquid state. This anomaly is crucial for aquatic life, as it prevents bodies of water from freezing solid from the bottom up in winter, allowing aquatic organisms to survive. The ice floats on the surface, insulating the water below.
Heat Capacity
Water also has a relatively high specific heat capacity, meaning it takes a considerable amount of heat energy to raise its temperature. This high heat capacity contributes to its ability to moderate temperatures, influencing climate and weather patterns. The heat absorbed during the melting of ice further contributes to this temperature-moderating effect.
Practical Applications of Water's Melting Point
Understanding the melting point of water is essential in numerous applications, including:
Food Preservation
Freezing food relies on lowering the temperature of water within the food to below 0°C, forming ice crystals. The rate of freezing and the size of ice crystals significantly affect the quality of the frozen food. Controlled freezing techniques are employed to minimize ice crystal formation and maintain the texture and quality of the food.
Cryopreservation
In cryopreservation, biological materials are frozen to extremely low temperatures to preserve them for long periods. The precise control of the freezing and thawing processes is crucial for preventing damage to the cells and tissues during the phase transitions.
Industrial Processes
Many industrial processes utilize the melting and freezing of water, such as ice making, refrigeration, and the production of various materials. Accurate temperature control is critical in these processes to ensure efficient and effective operation.
Environmental Monitoring
The melting and freezing of water play a critical role in environmental processes, including the formation of glaciers, sea ice, and snow cover. Monitoring these processes is crucial for understanding climate change and its impact on our planet.
Conclusion: The Significance of Water's Melting Point
The melting point of water at 0°C might seem like a simple fact, but it's a fundamental property with far-reaching consequences. Understanding the factors influencing this melting point, the anomalies exhibited by water, and its practical applications across various fields highlights its significance. From the preservation of food to cryopreservation techniques and the very dynamics of our planet’s climate, the melting point of water plays a crucial, often understated, role in shaping our world. Continued research into the intricacies of water's behavior at this transition point remains essential for numerous scientific and technological advancements. This understanding allows us to develop new technologies, improve existing processes, and better predict and respond to environmental changes. The seemingly simple number, 0°C, represents a complex interplay of forces and a cornerstone for understanding the unique and vital properties of water.
Latest Posts
Latest Posts
-
How Long Does It Take Mars To Orbit The Sun
Mar 15, 2025
-
How Many Meters Is 10 Ft
Mar 15, 2025
-
What Is 69 Inches In Feet
Mar 15, 2025
-
Lowest Common Multiple Of 3 And 4
Mar 15, 2025
-
Determine Charge On Capacitor In Following Circuit
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Melting Point Of Water In Celsius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
