Freezing Point For Water In Fahrenheit
Juapaving
Mar 26, 2025 · 5 min read
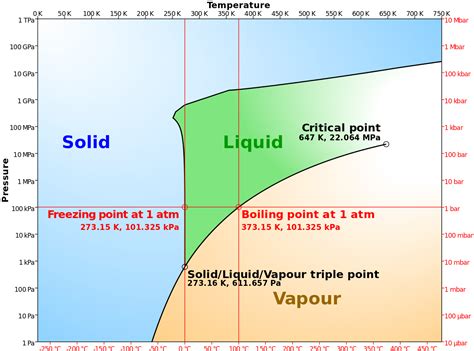
Table of Contents
Freezing Point of Water in Fahrenheit: A Deep Dive
The freezing point of water, a seemingly simple concept, holds significant importance across numerous scientific disciplines and everyday life. While we often learn that water freezes at 32° Fahrenheit (0° Celsius), a deeper understanding requires exploring the nuances impacting this critical temperature. This comprehensive guide delves into the freezing point of water in Fahrenheit, examining the underlying physics, factors influencing it, and its practical implications.
Understanding the Freezing Point
The freezing point, also known as the melting point, is the temperature at which a substance changes state from liquid to solid (freezing) or from solid to liquid (melting). For pure water under standard atmospheric pressure (one atmosphere), this transition occurs at precisely 32° Fahrenheit (32°F). This seemingly straightforward fact underpins many crucial processes in nature and technology.
The Role of Pressure
While 32°F is the standard freezing point, it's vital to understand that pressure influences this temperature. Increasing pressure slightly lowers the freezing point of water. This is an unusual property, unlike most substances where increased pressure raises the freezing point. This anomalous behavior is due to the unique structure of ice, where water molecules arrange themselves in a less dense crystalline structure than liquid water. This means that increasing pressure forces the less dense ice to transition into the denser liquid state, resulting in a lower freezing point. However, the pressure change needed to significantly alter the freezing point is considerable, and for most practical purposes, the 32°F value remains accurate.
The Impact of Impurities
The presence of impurities, such as dissolved salts or other substances, also affects the freezing point of water. This phenomenon is known as freezing point depression. The addition of solutes lowers the freezing point; the more solutes present, the greater the depression. This principle is widely used in applications like de-icing roads (using salt) and creating antifreeze solutions for car radiators. The dissolved salts disrupt the water molecules' ability to form a regular crystalline structure, requiring a lower temperature for freezing to occur.
Supercooling: A Curious Anomaly
Water can sometimes remain in a liquid state even below its freezing point – a phenomenon called supercooling. This occurs when the water lacks nucleation sites, which are microscopic imperfections or impurities that serve as starting points for ice crystal formation. In the absence of these sites, the water can remain in a metastable liquid state, but a slight disturbance, such as shaking the container or introducing a tiny ice crystal, will trigger immediate freezing.
Practical Applications of the Freezing Point
The 32°F freezing point of water is crucial across a vast range of applications:
Weather and Climate
Understanding the freezing point is fundamentally important in meteorology and climatology. Freezing temperatures determine the formation of snow, ice, and frost, which have profound effects on weather patterns, ecosystems, and infrastructure. Accurate prediction of freezing temperatures is essential for protecting crops, preventing infrastructure damage, and issuing severe weather warnings.
Food Preservation
Freezing is a common method of food preservation, leveraging the fact that water in food freezes at 32°F. Freezing slows down or halts the growth of microorganisms, extending the shelf life of food products. The precise control of freezing temperatures is critical to minimize ice crystal formation, which can damage the texture and quality of frozen food.
Industrial Processes
Many industrial processes rely on the freezing point of water. For example, the production of ice, ice cream, and other frozen goods requires precise temperature control to achieve the desired consistency and quality. Furthermore, many industrial processes use freezing as a method of separation or purification, leveraging the different freezing points of various substances.
Biology and Medicine
The freezing point of water is crucial in biological systems. Freezing temperatures can damage or destroy cells, which is why organisms have evolved various strategies for surviving freezing conditions. In medicine, cryopreservation, the preservation of cells, tissues, and organs at low temperatures, utilizes controlled freezing to maintain the viability of biological materials.
Factors Affecting the Accuracy of the 32°F Mark
While 32°F serves as a reliable benchmark, several subtle factors can influence the exact freezing point in real-world scenarios:
- Purity of water: As discussed earlier, impurities lower the freezing point. Even trace amounts of dissolved minerals can cause slight deviations.
- Pressure variations: While the effect is typically small at sea level, significant altitude changes or deep-sea pressure can alter the freezing point.
- Rate of cooling: Rapid cooling can lead to supercooling, delaying the freezing process.
- Presence of nucleation sites: The availability of surfaces or particles for ice crystal formation significantly influences freezing initiation.
Beyond 32°F: Exploring Related Concepts
The freezing point of water acts as a foundational concept for understanding other related phenomena:
- Boiling Point: The relationship between freezing and boiling points is crucial in understanding a substance's properties. Water boils at 212°F (100°C) at standard pressure.
- Triple Point: This is the unique temperature and pressure at which water can exist simultaneously in three phases: solid, liquid, and gas. For water, this point is approximately 0.01°C (32.018°F) and 611.657 pascals.
- Specific Heat: This is the amount of heat required to raise the temperature of a unit mass of a substance by one degree. Water has a relatively high specific heat, meaning it takes a considerable amount of energy to change its temperature. This influences its role in climate regulation.
Conclusion: The Enduring Significance of 32°F
The freezing point of water at 32°F is far more than a simple numerical value. It's a fundamental constant that underpins numerous natural processes and technological applications. Understanding its nuances, including the influence of pressure, impurities, and supercooling, provides a deeper appreciation of this critical temperature's significance in various fields from meteorology and food science to biology and industrial engineering. Continuing research and exploration in this area are essential for advancing our understanding of water's behavior and developing innovative applications in diverse sectors. The seemingly simple 32°F mark represents a complex and fascinating intersection of physics, chemistry, and the natural world.
Latest Posts
Latest Posts
-
What Is Depicted In The Image Above
Mar 29, 2025
-
14 Meters Is How Many Feet
Mar 29, 2025
-
How Many Electrons In Oxygen Atom
Mar 29, 2025
-
Whats The Prime Factorization Of 15
Mar 29, 2025
-
37 Inches Is How Many Feet
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point For Water In Fahrenheit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
