Force Per Unit Area Is Termed
Juapaving
Mar 31, 2025 · 6 min read
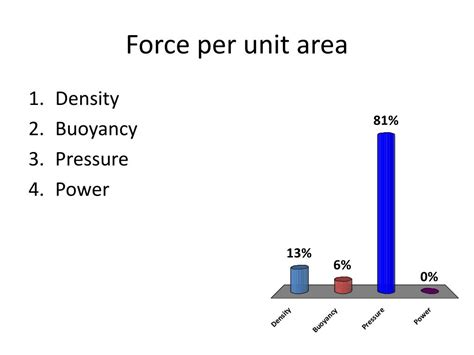
Table of Contents
Force per Unit Area is Termed: A Deep Dive into Pressure and its Applications
Force per unit area is a fundamental concept in physics and engineering, commonly termed pressure. Understanding pressure is crucial across numerous disciplines, from designing skyscrapers to understanding the behavior of fluids and gases. This article will explore pressure in detail, examining its definition, units of measurement, applications, and its relationship to other physical phenomena. We'll delve into various types of pressure, including hydrostatic pressure, atmospheric pressure, and gauge pressure, and discuss their practical significance.
Defining Pressure: Force Distributed Over an Area
Pressure is defined as the force exerted perpendicularly on a surface per unit area. This means that the pressure experienced at a point depends on both the magnitude of the force applied and the area over which that force is distributed. A smaller area subjected to the same force will experience a much higher pressure than a larger area. Mathematically, pressure (P) is expressed as:
P = F/A
where:
- P represents pressure
- F represents the force applied (in Newtons, N)
- A represents the area over which the force is applied (in square meters, m²)
Understanding the Units of Pressure
The SI unit for pressure is the Pascal (Pa), which is defined as one Newton per square meter (N/m²). However, various other units are commonly used, depending on the context:
- Pascal (Pa): The standard SI unit.
- Kilopascal (kPa): 1 kPa = 1000 Pa. Frequently used for meteorological purposes and in many engineering applications.
- Bar: 1 bar = 100,000 Pa. Used extensively in meteorology and some industrial settings.
- Atmosphere (atm): Approximately equal to the average atmospheric pressure at sea level (1 atm ≈ 101,325 Pa).
- Pounds per square inch (psi): A common unit in the United States and some other countries. 1 psi ≈ 6895 Pa.
- Millimeters of mercury (mmHg): Often used in medical applications to measure blood pressure. 1 mmHg ≈ 133.32 Pa.
The choice of unit depends largely on the magnitude of the pressure being measured and the specific application. For instance, atmospheric pressure is typically expressed in kPa or atm, while blood pressure is often measured in mmHg.
Types of Pressure: Exploring Different Contexts
Pressure manifests itself in various forms depending on the system under consideration. Let's explore some key types of pressure:
1. Hydrostatic Pressure: Pressure in Fluids at Rest
Hydrostatic pressure is the pressure exerted by a fluid at rest due to the weight of the fluid above it. The pressure increases with depth because the weight of the overlying fluid increases. The formula for hydrostatic pressure is:
P = ρgh
where:
- P is the hydrostatic pressure
- ρ is the density of the fluid
- g is the acceleration due to gravity
- h is the depth below the surface of the fluid
Hydrostatic pressure is crucial in understanding the behavior of liquids in containers, the pressure at the bottom of oceans and lakes, and the functioning of hydraulic systems.
2. Atmospheric Pressure: The Weight of the Air
Atmospheric pressure is the pressure exerted by the Earth's atmosphere. It's the result of the weight of the air column above a given point. Atmospheric pressure decreases with altitude because the weight of the overlying air decreases. This pressure difference is what drives weather systems and allows for the functioning of various instruments, such as barometers and altimeters.
Standard atmospheric pressure at sea level is approximately 101.3 kPa. However, this can vary depending on geographic location, altitude, and weather conditions.
3. Gauge Pressure: Pressure Relative to Atmospheric Pressure
Gauge pressure is the pressure measured relative to atmospheric pressure. It represents the difference between the absolute pressure and the atmospheric pressure. Many pressure gauges, such as those used to measure tire pressure or blood pressure, measure gauge pressure, not absolute pressure. The relationship between absolute pressure (P<sub>abs</sub>), gauge pressure (P<sub>gauge</sub>), and atmospheric pressure (P<sub>atm</sub>) is:
P<sub>abs</sub> = P<sub>gauge</sub> + P<sub>atm</sub>
4. Dynamic Pressure: Pressure in Moving Fluids
Dynamic pressure is the pressure associated with the motion of a fluid. It's a consequence of the fluid's kinetic energy and is directly proportional to the square of the fluid's velocity. Dynamic pressure plays a significant role in aerodynamics and fluid mechanics, influencing the lift generated by aircraft wings and the pressure drop in pipelines.
Applications of Pressure: A Diverse Range of Uses
Pressure is a fundamental quantity with applications across a vast range of fields:
1. Engineering and Design
- Hydraulic systems: Pressure is the driving force behind hydraulic systems, used in construction equipment, aircraft braking systems, and many industrial applications.
- Structural engineering: Engineers consider pressure when designing buildings, bridges, and other structures to ensure they can withstand the loads they are subjected to.
- Pipeline design: The design of pipelines for transporting liquids and gases must account for the pressure inside the pipes.
2. Meteorology and Climatology
- Weather forecasting: Atmospheric pressure is a crucial parameter in weather forecasting, used to predict changes in weather patterns.
- Altitude measurement: Altimeters use changes in atmospheric pressure to measure altitude.
3. Medicine
- Blood pressure measurement: Blood pressure is a critical indicator of health, measured using a sphygmomanometer.
- Respiratory therapy: Pressure is used in various respiratory therapy techniques, such as ventilators.
4. Industrial Processes
- Chemical processing: Pressure is often used to control chemical reactions and separation processes.
- Manufacturing: Pressure is employed in many manufacturing processes, such as molding and casting.
5. Everyday Life
- Tire pressure: Maintaining the correct tire pressure is important for safe and efficient driving.
- Water pressure: Water pressure in homes and businesses is crucial for supplying water to taps and appliances.
Pressure and its Relationship to Other Physical Phenomena
Pressure is intimately linked to other physical phenomena, such as:
- Temperature: Changes in temperature can affect the pressure of gases, as described by the ideal gas law (PV = nRT).
- Volume: For gases, pressure and volume are inversely related, as seen in Boyle's Law (at constant temperature).
- Density: Pressure in fluids is directly related to their density and depth.
Conclusion: A Forceful Concept with Widespread Impact
Pressure, the force per unit area, is a fundamental concept with profound implications across various scientific and engineering disciplines. From the design of massive structures to the functioning of the human body, understanding pressure is crucial for solving problems and advancing technological progress. The diverse units and forms of pressure, coupled with its relationship to other physical quantities, highlight its importance and its wide-ranging impact on the world around us. Continued research and advancements in the understanding of pressure will undoubtedly lead to even more innovative applications in the future. Further exploration into specific applications, such as the intricacies of fluid dynamics or the complexities of atmospheric science, will only deepen our appreciation for this essential physical quantity. Its pervasive influence underscores its fundamental role in shaping our physical reality.
Latest Posts
Latest Posts
-
Newtons First Law Examples In Everyday Life
Apr 01, 2025
-
15 4 As A Mixed Number
Apr 01, 2025
-
What Organelle Is The Site Of Aerobic Respiration
Apr 01, 2025
-
1 5 Gallons Is How Many Liters
Apr 01, 2025
-
How To Turn Gas Into Liquid
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Force Per Unit Area Is Termed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
