Electrical Conductivity Physical Or Chemical Property
Juapaving
Mar 28, 2025 · 6 min read
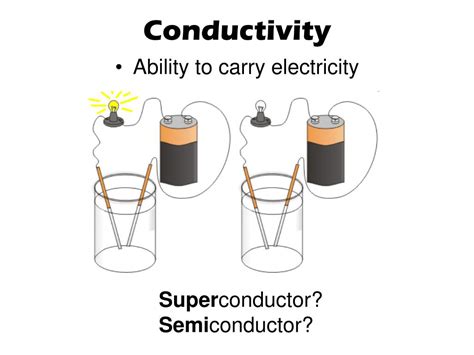
Table of Contents
Electrical Conductivity: A Deep Dive into a Fundamental Physical Property
Electrical conductivity, the ability of a material to conduct electric current, is a fundamental property with far-reaching implications across various scientific disciplines and technological applications. While often discussed alongside chemical properties, it's crucial to understand that electrical conductivity is fundamentally a physical property. This article will explore the intricacies of electrical conductivity, examining its underlying mechanisms, factors influencing it, and its significance in different material classes.
Understanding Electrical Conductivity: The Basics
Electrical conductivity quantifies how easily electric charge can flow through a material. It's the reciprocal of electrical resistivity, often represented by the Greek letter σ (sigma). High conductivity signifies that electrons or other charge carriers move freely, while low conductivity (high resistivity) indicates significant resistance to charge flow. The SI unit for electrical conductivity is siemens per meter (S/m).
The Role of Charge Carriers
The ability of a material to conduct electricity hinges on the availability and mobility of charge carriers. These are primarily electrons, but in certain materials, ions can also contribute to conductivity.
-
Metals: In metals, the outermost electrons are delocalized, forming a "sea" of electrons that are free to move throughout the material's structure. This abundance of mobile electrons gives metals their excellent electrical conductivity. The ease with which these electrons move is significantly affected by factors like temperature and the presence of impurities.
-
Semiconductors: Semiconductors exhibit an intermediate level of conductivity. Their conductivity is highly sensitive to temperature, impurities (doping), and electromagnetic radiation. In intrinsic semiconductors, the number of charge carriers is relatively low, but it increases dramatically with temperature as electrons gain enough energy to jump into the conduction band. Doping with impurities introduces additional charge carriers, dramatically altering their conductivity.
-
Insulators: Insulators possess very few mobile charge carriers. Their electrons are tightly bound to their respective atoms, making it extremely difficult for charge to flow. Consequently, insulators exhibit extremely low electrical conductivity.
Factors Influencing Electrical Conductivity
Several factors significantly influence a material's electrical conductivity:
1. Temperature: A Critical Factor
Temperature profoundly affects electrical conductivity, particularly in metals and semiconductors.
-
Metals: In metals, increased temperature leads to increased vibrational motion of the atoms in the lattice structure. These vibrations hinder the free movement of electrons, thus decreasing electrical conductivity. This relationship is often described by a negative temperature coefficient of resistance.
-
Semiconductors: In contrast, the temperature dependence of conductivity in semiconductors is significantly different. As temperature increases, more electrons gain sufficient energy to overcome the energy gap between the valence and conduction bands, increasing the number of charge carriers and boosting conductivity. This relationship is described by a positive temperature coefficient of resistance.
2. Material Composition and Structure
The atomic structure and composition of a material directly impact its electrical conductivity.
-
Crystalline Structure: Highly ordered crystalline structures tend to exhibit higher conductivity than amorphous materials because the regular arrangement of atoms facilitates easier electron movement. Crystal defects, such as vacancies or dislocations, can act as scattering centers, impeding electron flow and reducing conductivity.
-
Impurities and Alloys: The presence of impurities or alloying elements can significantly alter the electrical conductivity of a material. Some impurities can act as electron donors or acceptors, increasing or decreasing the number of charge carriers. Alloying can also influence the lattice structure and electron scattering, affecting conductivity.
3. Pressure: A Less Obvious Influence
Pressure can also influence electrical conductivity, particularly in solids. Applying pressure can alter the interatomic spacing, influencing electron mobility and subsequently conductivity. In some materials, pressure can induce phase transitions, resulting in dramatic changes in conductivity.
4. Presence of Magnetic Fields
Magnetic fields can also affect the movement of charge carriers, thereby influencing electrical conductivity. The effect is known as the Hall effect and is often used to determine the type of charge carriers (electrons or holes) in a material.
Electrical Conductivity in Different Material Classes
Electrical conductivity varies dramatically across different material classes, reflecting their unique atomic structures and bonding characteristics.
1. Metals: The Excellent Conductors
Metals are renowned for their exceptional electrical conductivity. This is attributed to the delocalized electrons in their metallic bonding, allowing for easy charge transport. Silver, copper, and gold are among the best electrical conductors. The conductivity of metals generally decreases with increasing temperature.
2. Semiconductors: The Versatile Middle Ground
Semiconductors exhibit conductivity intermediate between metals and insulators. Their conductivity is highly sensitive to temperature, doping, and other external factors. Silicon and germanium are prominent examples of semiconductors, crucial in modern electronics. The conductivity of semiconductors generally increases with increasing temperature.
3. Insulators: The Resistance Champions
Insulators possess extremely low electrical conductivity due to the tightly bound electrons in their atomic structure. These materials effectively block the flow of electric current. Common insulators include rubber, glass, and plastics. Their conductivity is typically minimally affected by temperature changes.
4. Superconductors: Zero Resistance
Superconductors are a special class of materials that exhibit zero electrical resistance below a critical temperature. This phenomenon allows for lossless transmission of electric current, making them highly desirable for various applications. However, achieving and maintaining the necessary low temperatures can be challenging.
Applications of Electrical Conductivity
Understanding and controlling electrical conductivity is crucial in countless technological applications.
-
Electronics: Semiconductors are the backbone of modern electronics, enabling the fabrication of transistors, integrated circuits, and other electronic components. The precise control over conductivity through doping is essential for their functionality.
-
Power Transmission: High-conductivity metals like copper and aluminum are extensively used in power transmission lines to minimize energy loss during electricity distribution.
-
Sensors: Changes in electrical conductivity can be used to sense various physical and chemical phenomena, leading to the development of conductivity-based sensors for temperature, humidity, gas detection, and more.
-
Electroplating: The process of electroplating relies on the principle of electrical conductivity to deposit a thin layer of metal onto a substrate.
-
Materials Science: The measurement of electrical conductivity is an important characterization technique in materials science, providing insights into the material's structure, composition, and properties.
Conclusion: The Significance of a Physical Property
Electrical conductivity, a fundamental physical property, plays a pivotal role in shaping our technological landscape. Its sensitivity to various factors allows for its precise control and manipulation, leading to advancements across diverse fields. Further research and development in materials science are continuously striving to uncover new materials with enhanced or tailored electrical conductivity properties, promising exciting technological breakthroughs in the future. Understanding the intricate interplay of factors influencing electrical conductivity remains crucial for developing innovative solutions in areas ranging from electronics and energy to sensing and materials science. The exploration of this physical property continues to be a cornerstone of scientific progress and technological innovation.
Latest Posts
Latest Posts
-
How Many Meters In 7 Feet
Mar 31, 2025
-
How Is A Square Similar To A Rhombus
Mar 31, 2025
-
Work Done For The Process Shown In The Figure Is
Mar 31, 2025
-
Write The Electron Configuration For A Neutral Atom Of Germanium
Mar 31, 2025
-
What Two Numbers Multiply To 32
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Electrical Conductivity Physical Or Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
