Diffusion Rate Is Fastest When The Concentration Gradient Is
Juapaving
Mar 16, 2025 · 5 min read
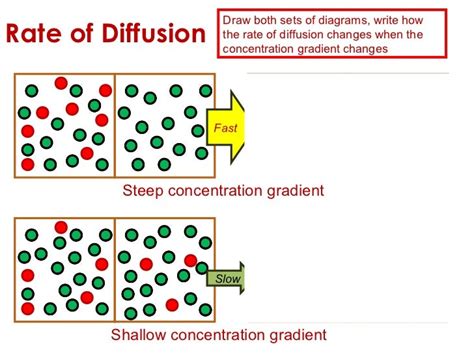
Table of Contents
Diffusion Rate is Fastest When the Concentration Gradient is Steepest
Diffusion, the spontaneous movement of particles from an area of high concentration to an area of low concentration, is a fundamental process in many areas of science, from biology and chemistry to materials science and engineering. Understanding the factors that influence the rate of diffusion is crucial for optimizing various applications, from drug delivery to the design of efficient fuel cells. A key factor determining the speed of diffusion is the concentration gradient. This article delves deep into the relationship between concentration gradient and diffusion rate, exploring the underlying principles and providing real-world examples.
Understanding Concentration Gradient
The concentration gradient describes the difference in concentration of a substance between two points. Imagine a cup of tea with a sugar cube dropped into it. Initially, the sugar concentration is extremely high near the cube and very low further away. This difference creates a concentration gradient. The steeper the gradient (the bigger the difference in concentration between two points), the faster the sugar molecules will diffuse throughout the tea.
Visualizing the Gradient:
Think of a hill. The steepness of the hill represents the concentration gradient. A steep hill (large concentration gradient) means a rapid descent (fast diffusion). A gentle slope (small concentration gradient) signifies a slow descent (slow diffusion).
Mathematical Representation:
The concentration gradient is often expressed as a derivative in calculus: dC/dx, where 'C' represents concentration and 'x' represents distance. A large positive value of dC/dx indicates a steep concentration gradient, and vice-versa.
Fick's First Law of Diffusion: The Quantitative Relationship
The relationship between diffusion rate and concentration gradient is precisely described by Fick's First Law of Diffusion:
J = -D (dC/dx)
Where:
- J represents the diffusion flux (the amount of substance diffusing per unit area per unit time). It's a measure of the diffusion rate.
- D is the diffusion coefficient, a constant that depends on the diffusing substance, the medium it's diffusing through, and the temperature.
- dC/dx is the concentration gradient, as previously defined.
The negative sign indicates that diffusion occurs in the direction of decreasing concentration. Crucially, this equation demonstrates a direct proportionality between the diffusion flux (rate) and the concentration gradient. A steeper gradient (larger dC/dx) leads to a higher diffusion flux (faster diffusion).
Factors Affecting Diffusion Coefficient (D)
While the concentration gradient is the primary driver of diffusion rate, the diffusion coefficient (D) also plays a crucial role. Several factors influence D:
1. Temperature:
Higher temperatures generally lead to higher diffusion coefficients. Increased thermal energy provides molecules with more kinetic energy, enabling them to move more rapidly and overcome intermolecular forces, thus facilitating faster diffusion.
2. Medium Properties:
The medium through which diffusion occurs significantly affects the diffusion coefficient. Diffusion is faster in less dense media (like gases) compared to denser media (like solids). The viscosity and porosity of the medium also play critical roles. For instance, diffusion through a porous membrane will be slower than diffusion through free space.
3. Molecular Size and Shape:
Smaller molecules generally diffuse faster than larger molecules. Larger molecules experience more friction and collisions as they move through the medium, hindering their diffusion. The shape of the molecule also plays a role, with more streamlined shapes diffusing more efficiently.
4. Interactions between Diffusing Molecules and the Medium:
The nature of interactions between the diffusing molecules and the medium influences the diffusion coefficient. Stronger interactions (e.g., hydrogen bonding) can slow down diffusion.
Real-World Examples of Diffusion Rate and Concentration Gradient
The relationship between diffusion rate and concentration gradient is observable in numerous real-world scenarios:
1. Oxygen Uptake in Lungs:
The efficient uptake of oxygen from the alveoli (air sacs) in the lungs into the bloodstream relies on a steep concentration gradient. The high oxygen concentration in the alveoli and the low oxygen concentration in the blood create a strong driving force for oxygen diffusion across the alveolar-capillary membrane.
2. Nutrient Absorption in the Intestine:
The absorption of nutrients from the digested food in the small intestine depends on the concentration gradient between the intestinal lumen and the blood capillaries. Efficient digestion and absorption are facilitated by maintaining a steep concentration gradient.
3. Drug Delivery:
Pharmaceutical scientists carefully design drug delivery systems to ensure that drugs reach their target tissues effectively. This often involves controlling the concentration gradient to optimize drug release and absorption. For instance, sustained-release formulations aim to maintain a relatively constant concentration gradient over time.
4. Semiconductor Manufacturing:
In the semiconductor industry, diffusion plays a crucial role in doping processes. Precise control over the concentration gradient of dopant atoms is essential for creating the desired electrical properties in semiconductor devices.
5. Environmental Science:
Understanding diffusion is critical in studying the spread of pollutants in the environment. The concentration gradient dictates how quickly pollutants disperse, impacting the extent and severity of environmental contamination.
Enhancing Diffusion: Practical Implications
Several strategies can be employed to enhance diffusion rates:
- Increasing the concentration gradient: This is the most direct method of speeding up diffusion. Maintaining a large difference in concentration between the source and destination areas will drive faster diffusion.
- Increasing temperature: As explained earlier, higher temperatures increase the kinetic energy of molecules, accelerating diffusion.
- Reducing the viscosity of the medium: Lower viscosity allows molecules to move more freely, leading to faster diffusion.
- Modifying the medium to increase porosity: Creating a more porous medium will facilitate faster diffusion, as molecules have more pathways to travel.
- Reducing molecular size: Using smaller molecules will generally result in faster diffusion.
Conclusion:
The concentration gradient is the primary driving force behind diffusion. The steeper the concentration gradient, the faster the rate of diffusion. Fick's First Law of Diffusion quantitatively describes this relationship. Understanding this relationship is essential across various scientific and engineering disciplines. Optimizing diffusion processes involves manipulating factors that influence both the concentration gradient and the diffusion coefficient to achieve desired outcomes in diverse applications ranging from biological systems to industrial processes. By controlling these factors, we can enhance the efficiency and effectiveness of many processes that rely on diffusion. Continued research and innovation in this area promise further advancements in various fields, improving our understanding and ability to harness the power of diffusion.
Latest Posts
Latest Posts
-
What Is An Operator In Biology
Mar 17, 2025
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Diffusion Rate Is Fastest When The Concentration Gradient Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
