Difference Between Nuclear Reaction And Chemical Reaction
Juapaving
Mar 25, 2025 · 6 min read
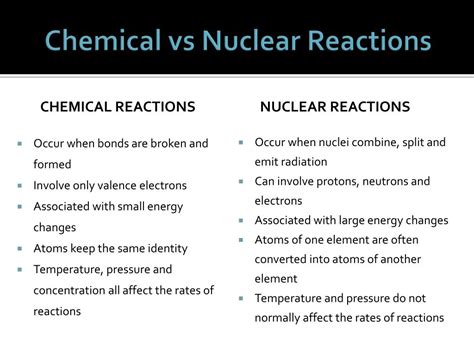
Table of Contents
Delving Deep into the Differences: Nuclear Reactions vs. Chemical Reactions
Understanding the fundamental differences between nuclear reactions and chemical reactions is crucial for comprehending the world around us. While both involve energy transformations and changes in matter, the scale and nature of these changes differ dramatically. This article will explore the core distinctions, examining the types of reactions, energy involved, changes in matter, and real-world applications of each.
Key Differences: A Bird's-Eye View
Before diving into the specifics, let's establish the primary differences between nuclear and chemical reactions:
-
Type of Change: Chemical reactions involve changes in the arrangement of electrons within and between atoms, resulting in the formation or breaking of chemical bonds. Nuclear reactions, on the other hand, involve changes in the nucleus of an atom, altering the number of protons and neutrons. This fundamental difference is the cornerstone of their distinct characteristics.
-
Energy Involved: Nuclear reactions involve vastly greater energy changes compared to chemical reactions. This is because the strong nuclear force binding protons and neutrons in the nucleus is significantly stronger than the electromagnetic force responsible for chemical bonding. Nuclear reactions release or absorb significantly more energy.
-
Matter Transformation: Chemical reactions rearrange atoms to form new molecules, but the atoms themselves remain unchanged. Nuclear reactions, however, can transform one element into another (transmutation), fundamentally changing the atomic identity.
-
Rate of Reaction: Chemical reactions typically occur at much faster rates compared to nuclear reactions. While some chemical reactions can be slow, nuclear reactions often proceed at slower rates and are often difficult to control.
-
Reaction Products: Chemical reactions produce new compounds with altered chemical properties, but the atoms involved remain the same. Nuclear reactions, conversely, can create entirely new elements or isotopes, often resulting in radioactive products.
Chemical Reactions: A Deep Dive
Chemical reactions are the foundation of chemistry, encompassing countless processes that govern the interactions between atoms and molecules. These reactions involve the breaking and forming of chemical bonds, resulting in the rearrangement of atoms and the formation of new substances.
Types of Chemical Reactions
Chemical reactions are categorized based on several factors, including:
-
Combination (Synthesis) Reactions: Two or more reactants combine to form a single product (e.g., 2H₂ + O₂ → 2H₂O).
-
Decomposition Reactions: A single reactant breaks down into two or more products (e.g., 2H₂O → 2H₂ + O₂).
-
Single Displacement (Substitution) Reactions: One element replaces another in a compound (e.g., Zn + 2HCl → ZnCl₂ + H₂).
-
Double Displacement (Metathesis) Reactions: Two compounds exchange ions to form two new compounds (e.g., AgNO₃ + NaCl → AgCl + NaNO₃).
-
Acid-Base Reactions (Neutralization): An acid reacts with a base to form salt and water (e.g., HCl + NaOH → NaCl + H₂O).
-
Redox (Oxidation-Reduction) Reactions: Involve the transfer of electrons between reactants, with one species undergoing oxidation (loss of electrons) and the other undergoing reduction (gain of electrons).
Energy Changes in Chemical Reactions
Chemical reactions can be either exothermic (releasing energy) or endothermic (absorbing energy). Exothermic reactions, like combustion, release heat to their surroundings. Endothermic reactions, like photosynthesis, absorb heat from their surroundings. The energy changes involved in chemical reactions are relatively small compared to those in nuclear reactions. This energy difference is often measured in kilojoules (kJ) per mole of reactant.
Nuclear Reactions: Unraveling the Atom's Core
Nuclear reactions involve changes within the atom's nucleus, affecting the number of protons and neutrons. These reactions release or absorb enormous amounts of energy, far surpassing the energy changes seen in chemical reactions.
Types of Nuclear Reactions
Several types of nuclear reactions are significant:
-
Nuclear Fission: The splitting of a heavy atomic nucleus into two or more lighter nuclei, releasing a tremendous amount of energy. This is the principle behind nuclear power plants and atomic bombs. A classic example is the fission of uranium-235.
-
Nuclear Fusion: The combining of two light atomic nuclei to form a heavier nucleus, also releasing a vast amount of energy. This process powers the sun and other stars. Fusion reactions require extremely high temperatures and pressures. An example is the fusion of deuterium and tritium to form helium.
-
Radioactive Decay: The spontaneous emission of particles or energy from an unstable atomic nucleus. This process transforms the unstable nucleus into a more stable one. Several types of decay exist, including alpha decay, beta decay, and gamma decay.
-
Nuclear Transmutation: The conversion of one element into another through bombardment with particles like neutrons or protons. This process is used to create artificial isotopes and radioisotopes for various applications.
Energy Changes in Nuclear Reactions
Nuclear reactions involve energy changes on a scale vastly exceeding those in chemical reactions. The energy released or absorbed is typically measured in megaelectronvolts (MeV) per nucleus, a unit significantly larger than the kilojoules used for chemical reactions. This immense energy difference accounts for the devastating power of nuclear weapons and the potential of nuclear energy for electricity generation.
Comparing the Two: A Table Summary
| Feature | Chemical Reaction | Nuclear Reaction |
|---|---|---|
| Type of Change | Rearrangement of electrons, bonding changes | Changes in the atomic nucleus (protons and neutrons) |
| Energy Involved | Relatively small (kJ/mol) | Extremely large (MeV/nucleus) |
| Matter Transformation | No change in atomic identity | Possible transmutation of elements |
| Rate of Reaction | Typically fast | Typically slow |
| Reaction Products | New compounds with different chemical properties | New elements or isotopes, often radioactive |
| Examples | Combustion, rusting, photosynthesis | Fission, fusion, radioactive decay |
Real-World Applications
Both chemical and nuclear reactions have profound impacts on our lives:
Chemical Reactions in Everyday Life:
- Combustion: Fuels our cars, heats our homes, and powers many industrial processes.
- Photosynthesis: Provides the basis for almost all food chains on Earth.
- Respiration: Enables living organisms to extract energy from food.
- Synthesis of materials: Enables the creation of plastics, pharmaceuticals, and many other essential materials.
Nuclear Reactions in Everyday Life:
- Nuclear Power Generation: Provides a significant source of electricity in some countries.
- Medical Applications: Used in cancer treatment (radiotherapy), medical imaging (PET scans), and sterilization.
- Industrial Applications: Used in gauging thickness, tracing materials, and preserving food.
- Carbon Dating: Used to determine the age of ancient artifacts.
Conclusion: Understanding the Nuances
The distinction between chemical and nuclear reactions is fundamental to understanding the physical world. While both involve transformations of matter and energy, the scale and nature of these transformations are dramatically different. Chemical reactions involve rearrangements of electrons and bonds, while nuclear reactions involve changes in the nucleus, capable of transmuting elements and releasing vastly greater energies. By understanding these core differences, we can appreciate the power and potential of both chemical and nuclear processes in shaping our world, from the everyday to the extraordinary. The continued study of both is crucial for technological advancement and a deeper comprehension of the universe around us.
Latest Posts
Latest Posts
-
Balanced Equation For Na And H2o
Mar 25, 2025
-
What Was The Melting Point Of Water
Mar 25, 2025
-
What Is The Common Factor Of 16 And 24
Mar 25, 2025
-
What Is The Largest Cell Of The Human Body
Mar 25, 2025
-
Common Multiples Of 12 And 16
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Nuclear Reaction And Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
