Difference Between Lewis Acid And Bronsted Acid
Juapaving
Mar 25, 2025 · 5 min read
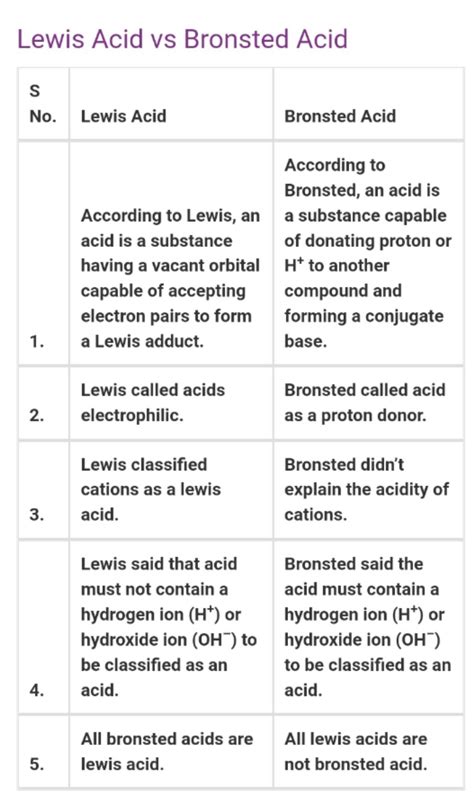
Table of Contents
Delving Deep: Unveiling the Differences Between Lewis and Brønsted Acids
Acids are fundamental chemical entities playing crucial roles in countless reactions. While the concept of acids might seem straightforward, the definition itself boasts nuances and complexities. Two prominent acid classifications, Brønsted-Lowry and Lewis, offer distinct yet interconnected perspectives on acidity. Understanding their differences is key to mastering acid-base chemistry. This comprehensive article meticulously explores the fundamental differences between Lewis acids and Brønsted-Lowry acids, delving into their definitions, examples, and applications.
Defining the Players: Brønsted-Lowry vs. Lewis Acids
The cornerstone of acid-base chemistry lies in the definition of acids and bases. Two prominent definitions dominate the landscape: the Brønsted-Lowry and the Lewis definitions. Let's dissect each:
Brønsted-Lowry Acids: The Proton Donors
A Brønsted-Lowry acid is defined as a substance that donates a proton (H⁺) in a chemical reaction. This definition is centered around the transfer of a hydrogen ion, also known as a proton. The key characteristic here is the ability of the acid to release a proton, leaving behind its conjugate base. The conjugate base is the species that remains after the acid has donated its proton.
Examples of Brønsted-Lowry Acids:
- Hydrochloric acid (HCl): HCl readily donates a proton, forming the chloride ion (Cl⁻) and a hydronium ion (H₃O⁺) in aqueous solution. This is a classic example of a strong Brønsted-Lowry acid.
- Acetic acid (CH₃COOH): Acetic acid, a weak acid, partially donates a proton to water, forming the acetate ion (CH₃COO⁻) and hydronium ions. The equilibrium lies significantly towards the undissociated acid, signifying its weak nature.
- Sulfuric acid (H₂SO₄): This is a strong diprotic acid, meaning it can donate two protons in stepwise reactions.
Lewis Acids: The Electron Pair Acceptors
A Lewis acid takes a broader perspective on acidity. Instead of focusing on proton donation, the Lewis definition centers on the acceptance of an electron pair. A Lewis acid is any substance capable of accepting an electron pair from a Lewis base. This significantly expands the scope of acidity beyond proton-donating species.
Examples of Lewis Acids:
- Boron trifluoride (BF₃): BF₃ possesses an incomplete octet, making it electron-deficient and readily accepting an electron pair from a Lewis base.
- Aluminum chloride (AlCl₃): Similar to BF₃, AlCl₃ acts as a Lewis acid due to its electron deficiency.
- Iron(III) ion (Fe³⁺): Transition metal cations often act as Lewis acids due to their high positive charge, which attracts electron pairs.
- Carbon dioxide (CO₂): While not immediately obvious, the carbon atom in CO₂ can accept an electron pair, making it a Lewis acid.
Key Differences: A Comparative Analysis
The fundamental difference between Brønsted-Lowry and Lewis acids lies in their mechanism of action:
| Feature | Brønsted-Lowry Acid | Lewis Acid |
|---|---|---|
| Definition | Proton (H⁺) donor | Electron pair acceptor |
| Mechanism | Proton transfer | Electron pair acceptance |
| Scope | Limited to proton-containing species | Includes a wider range of substances |
| Examples | HCl, CH₃COOH, H₂SO₄ | BF₃, AlCl₃, Fe³⁺, CO₂ |
| Conjugate Base | Formed after proton donation | Not directly applicable in the same manner |
Beyond the Proton: Expanding the Acidic Landscape
The Lewis definition significantly broadens the concept of acidity. Many substances that don't fit the Brønsted-Lowry definition are readily classified as Lewis acids. This inclusiveness is a significant advantage, allowing for a more comprehensive understanding of acid-base reactions in diverse chemical systems.
For instance, consider the reaction between boron trifluoride (BF₃) and ammonia (NH₃). BF₃, a Lewis acid, accepts an electron pair from the nitrogen atom in ammonia (a Lewis base), forming a coordinate covalent bond. This reaction does not involve proton transfer, making it impossible to classify using the Brønsted-Lowry definition.
Illustrative Examples and Applications
Let's explore a few examples to illustrate the differences further:
Example 1: Reaction of HCl with Water
HCl + H₂O → H₃O⁺ + Cl⁻
In this reaction, HCl (Brønsted-Lowry acid) donates a proton to water, forming the hydronium ion (H₃O⁺). This is a classic Brønsted-Lowry acid-base reaction. HCl also acts as a Lewis acid, accepting an electron pair from the oxygen atom in water. However, the proton transfer aspect is more prominently emphasized in this case.
Example 2: Reaction of BF₃ with NH₃
BF₃ + NH₃ → F₃B-NH₃
Here, BF₃ (Lewis acid) accepts an electron pair from the nitrogen atom in NH₃ (Lewis base), forming a coordinate covalent bond. This reaction does not involve proton transfer and, therefore, cannot be described using the Brønsted-Lowry model.
Example 3: The Friedel-Crafts Acylation
The Friedel-Crafts acylation is an important organic reaction that utilizes Lewis acids as catalysts. Aluminum chloride (AlCl₃), a Lewis acid, facilitates the reaction by accepting an electron pair from the carbonyl group of the acyl chloride, making it more electrophilic and susceptible to attack by an aromatic ring. This reaction would not proceed efficiently without the Lewis acid catalyst.
Overlap and Interrelation: Not Mutually Exclusive
While the definitions differ, it's crucial to recognize that the Brønsted-Lowry and Lewis definitions are not mutually exclusive. All Brønsted-Lowry acids are also Lewis acids. This is because the proton (H⁺) itself is a Lewis acid – it accepts an electron pair from the base during proton transfer. However, the reverse is not true: many Lewis acids are not Brønsted-Lowry acids.
Conclusion: A Unified Perspective
The Brønsted-Lowry and Lewis definitions provide complementary perspectives on acidity. The Brønsted-Lowry definition offers a simpler, more readily grasped concept focused on proton transfer, particularly useful in introductory chemistry. However, the Lewis definition provides a significantly broader framework, encompassing a much wider range of chemical species and reactions, crucial for understanding complex chemical systems. Mastering both definitions is essential for a comprehensive understanding of acid-base chemistry and its applications in various fields. By appreciating the nuances of each definition and recognizing their interrelationship, chemists gain a powerful toolset for analyzing and predicting the behavior of acids and bases in diverse chemical environments. This understanding is fundamental to progress in various areas such as catalysis, synthesis, and material science.
Latest Posts
Latest Posts
-
Write 28 As A Product Of Prime Factors
Mar 28, 2025
-
Which Expression Is Equivalent To Sqrt 200
Mar 28, 2025
-
Is N More Electronegative Than C
Mar 28, 2025
-
Lcm Of 3 7 And 10
Mar 28, 2025
-
A Tuning Fork Of Frequency 440 Hz
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Lewis Acid And Bronsted Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
