Difference Between Equivalence Point And End Point
Juapaving
Mar 16, 2025 · 6 min read
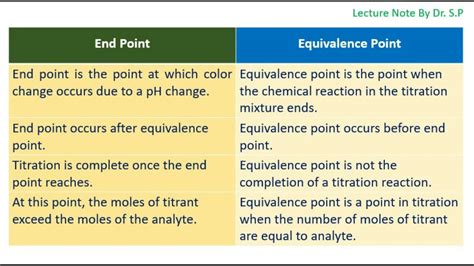
Table of Contents
The Difference Between Equivalence Point and Endpoint in Titration
Titration is a fundamental technique in analytical chemistry used to determine the concentration of an unknown solution (analyte) by reacting it with a solution of known concentration (titrant). Understanding the concepts of the equivalence point and the endpoint is crucial for accurate and reliable titration results. While often used interchangeably, these two points represent distinct stages in the titration process and their difference can significantly impact the accuracy of the analysis. This article will delve deep into the nuances of both, clarifying the distinction and explaining their importance in various titration applications.
Understanding the Equivalence Point
The equivalence point is the theoretical point in a titration where the amount of titrant added is stoichiometrically equivalent to the amount of analyte present. In simpler terms, it's the point at which the moles of titrant exactly react with the moles of analyte, according to the balanced chemical equation of the reaction. This point represents the complete neutralization or reaction between the titrant and the analyte.
Key Characteristics of the Equivalence Point:
- Stoichiometrically Defined: It's determined solely by the balanced chemical equation and the molar quantities of the reactants.
- Theoretical Point: It's a theoretical point, not directly observable during the titration. We infer its occurrence based on the data obtained.
- Precise: It represents the exact point of complete reaction.
- Dependent on Reaction Stoichiometry: The equivalence point's location varies based on the molar ratio of reactants in the balanced equation. For example, a reaction with a 1:1 molar ratio will have a different equivalence point than one with a 2:1 molar ratio.
Determining the Equivalence Point:
The equivalence point isn't directly observed during the titration. Instead, it's determined indirectly through methods such as:
- Plotting a Titration Curve: A graph of the pH (or other relevant property) versus the volume of titrant added. The equivalence point is identified as the point of steepest slope or inflection point on the curve. This is particularly useful for acid-base titrations.
- Calculating from Stoichiometry: Using the known concentration and volume of the titrant and the balanced chemical equation, the volume of titrant required to reach the equivalence point can be calculated.
- Using an Indicator (Indirectly): While indicators help determine the endpoint, they provide an approximation of the equivalence point. The closer the endpoint to the equivalence point, the more accurate the titration.
Understanding the Endpoint
The endpoint is the point in a titration where a noticeable change occurs, signaling that the reaction is essentially complete. This change is typically observed visually, often using an indicator that changes color upon reaching a specific pH or condition.
Key Characteristics of the Endpoint:
- Observable Change: It's characterized by a readily visible change, such as a color change in an indicator.
- Practical Point: It's a practical point, directly observable during the titration.
- Approximate: It's an approximation of the equivalence point, not the exact point of complete reaction.
- Indicator Dependent: The endpoint is influenced by the choice of indicator and its properties. Different indicators have different transition ranges, leading to variations in the endpoint.
Choosing and Using Indicators:
The selection of an appropriate indicator is crucial for obtaining an accurate endpoint. The indicator's color change should ideally occur very close to the equivalence point. Factors to consider include:
- pH Range: The indicator's pKa should be close to the pH at the equivalence point.
- Sharpness of Color Change: A sharp and distinct color change facilitates precise endpoint determination.
- Sensitivity: The indicator should be sensitive enough to detect the change in pH or other relevant property at or near the equivalence point.
The Difference Between Equivalence Point and Endpoint: A Critical Distinction
The key difference lies in their nature: the equivalence point is a theoretical concept based on stoichiometry, while the endpoint is a practical observation based on visual changes. The endpoint is an approximation of the equivalence point. The closer the endpoint is to the equivalence point, the more accurate the titration result will be.
Sources of Error:
Several factors can lead to discrepancies between the equivalence point and endpoint:
- Indicator Error: The indicator's transition range might not perfectly overlap with the equivalence point pH.
- Improper Technique: Insufficient mixing, slow addition of titrant, or parallax error in reading the burette can affect the endpoint determination.
- Temperature Effects: Changes in temperature can affect the reaction equilibrium and hence the equivalence point and endpoint.
- Competing Reactions: Presence of other reacting species might interfere with the main titration reaction, leading to errors.
Minimizing the Difference:
Several strategies can be employed to minimize the difference between the equivalence point and the endpoint:
- Careful Indicator Selection: Choosing an indicator with a transition range close to the expected equivalence point pH.
- Appropriate Titration Technique: Using proper mixing techniques, adding titrant slowly near the endpoint, and avoiding parallax error in burette readings.
- Temperature Control: Maintaining a constant temperature throughout the titration.
- Using a Potentiometric Titration: Instead of visual indicators, utilizing a pH meter or other potentiometric methods can provide a more accurate determination of the equivalence point.
Examples Illustrating the Difference
Let's consider some specific examples to further clarify the difference:
1. Strong Acid-Strong Base Titration:
In the titration of a strong acid (e.g., HCl) with a strong base (e.g., NaOH), the equivalence point occurs at pH 7. An indicator like phenolphthalein (pH range 8.2-10.0) would be suitable, resulting in an endpoint near pH 9. While there is a difference, it's relatively small in this case, leading to reasonably accurate results.
2. Weak Acid-Strong Base Titration:
Titrating a weak acid (e.g., acetic acid) with a strong base will have an equivalence point at a pH greater than 7 due to the conjugate base's hydrolysis. Choosing an indicator like phenolphthalein might lead to a significant difference between the endpoint and equivalence point because the pH change around the equivalence point is less sharp compared to a strong acid-strong base titration.
3. Redox Titration:
In redox titrations, the equivalence point is identified by the complete oxidation or reduction of the analyte. The endpoint might be detected through a change in color of the analyte itself or the use of a redox indicator. The difference between the equivalence point and endpoint would depend on the chosen indicator's sensitivity and the sharpness of the redox potential change at the equivalence point.
Conclusion
The equivalence point and endpoint are distinct yet related concepts in titration. Understanding their differences is crucial for achieving accurate results. While the equivalence point is a theoretical concept based on stoichiometry, the endpoint is a practical observation often reliant on an indicator's visual change. Minimizing the difference between these two points through careful technique, indicator selection, and, where appropriate, instrumental methods is crucial for ensuring the reliability and accuracy of quantitative analysis via titration. The closer the endpoint matches the equivalence point, the more precise the determined concentration of the unknown analyte will be. Continuous awareness and careful execution are key to successful titrations and accurate results.
Latest Posts
Latest Posts
-
What Is An Operator In Biology
Mar 17, 2025
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Equivalence Point And End Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
