Difference Between A Soap And A Detergent
Juapaving
Mar 12, 2025 · 6 min read
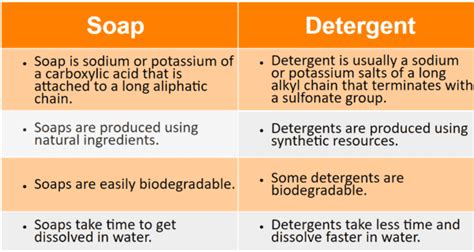
Table of Contents
The Great Soap vs. Detergent Debate: Understanding the Differences
For centuries, cleaning has been a fundamental aspect of human life. While the methods and tools have evolved dramatically, the core purpose remains the same: to remove dirt, grime, and unwanted substances. But within the world of cleaning agents, a constant source of confusion exists: the difference between soap and detergent. While both accomplish the task of cleaning, understanding their fundamental differences is key to choosing the right product for various cleaning needs. This comprehensive guide dives deep into the chemistry, properties, and applications of soaps and detergents, helping you make informed decisions for a cleaner, more efficient household.
The Chemistry Behind the Clean: Soap vs. Detergent
The core distinction between soap and detergent lies in their chemical composition and how they interact with water and dirt.
Soaps: The Natural Clean
Soaps are naturally derived cleaning agents produced through a process called saponification. This involves reacting fats or oils (typically animal fats or vegetable oils) with a strong alkali, such as lye (sodium hydroxide) or potassium hydroxide. This chemical reaction breaks down the fats into fatty acids, forming a salt—the soap. The type of fat or oil used determines the properties of the resulting soap, influencing its lather, hardness, and cleaning power.
Key characteristics of soaps:
- Biodegradable: Soaps are generally biodegradable, meaning they break down naturally in the environment without causing significant pollution.
- pH-balanced: Soaps tend to be less harsh than detergents, making them gentler on skin and fabrics.
- Water-hardness sensitivity: This is a crucial point. Soaps react with hard water (water containing high levels of minerals like calcium and magnesium) to form insoluble precipitates called soap scum. This reduces their cleaning effectiveness and leaves a residue.
- Mild cleaning power: While effective for many cleaning tasks, soaps generally possess a milder cleaning power compared to detergents.
Detergents: The Synthetic Solution
Detergents, on the other hand, are synthetically produced cleaning agents. They are manufactured from petrochemicals or other raw materials, and their chemical structure differs significantly from soaps. Detergents are typically composed of surfactants, which are molecules with both water-loving (hydrophilic) and oil-loving (hydrophobic) ends. This unique structure enables them to emulsify and suspend dirt and grime in water, facilitating their removal.
Key characteristics of detergents:
- Effective in hard water: Unlike soaps, detergents are less affected by hard water, maintaining their cleaning efficiency even in the presence of minerals.
- Stronger cleaning power: Detergents generally possess a stronger cleaning power compared to soaps, making them effective at removing stubborn stains and grease.
- Variety of formulations: Detergents are available in a vast array of formulations, tailored for specific cleaning tasks, such as laundry, dishwashing, and floor cleaning.
- Biodegradability varies: The biodegradability of detergents varies depending on their chemical composition. Older formulations were less environmentally friendly, but modern detergents are largely designed to be biodegradable.
Comparing Cleaning Mechanisms: How They Remove Dirt
Both soaps and detergents work by reducing the surface tension of water, allowing it to penetrate fabrics and surfaces more effectively. However, their mechanisms for removing dirt differ slightly.
Soap's Cleaning Action: Emulsification and Micelle Formation
Soap molecules form micelles in water. A micelle is a spherical structure with the hydrophobic tails pointing inwards and the hydrophilic heads facing outwards. When soap encounters dirt or grease (which is hydrophobic), the hydrophobic tails of the soap molecules surround the dirt, forming a micelle that encapsulates the dirt particle. This process, called emulsification, traps the dirt within the micelle, preventing it from reattaching to the surface. The micelles are then easily rinsed away with water, leaving the surface clean.
Detergent's Cleaning Action: Surfactant Power
Detergents utilize surfactants to achieve cleaning. Surfactants work similarly to soap by forming micelles, but their chemical structure allows for better performance in hard water. The surfactant molecules effectively lower the surface tension of water, enabling better wetting and penetration of fabrics and surfaces. Furthermore, some detergents contain enzymes that break down specific types of stains, enhancing their cleaning effectiveness. The combination of surfactant action and potential enzymatic activity makes detergents highly effective at removing a wider range of soils than soap alone.
Application Specifics: Choosing the Right Clean
The choice between soap and detergent often depends on the specific cleaning task:
Laundry: Detergents Reign Supreme
For laundry, detergents are generally preferred. Their superior cleaning power, especially in hard water, ensures effective stain removal and brighter clothes. Detergents are formulated to tackle a wide range of soils and stains, providing more consistent results than soap.
Dishwashing: A Balanced Approach
Both soap and detergent are used for dishwashing. However, dishwashing detergents are specifically designed for this purpose and often contain ingredients that enhance grease cutting and prevent spotting. While soap can be effective, it may leave residue in hard water.
Body Cleansing: Soap's Gentle Touch
For body cleansing, soap is often preferred due to its milder nature. While some detergents are formulated for personal use, many can be too harsh for delicate skin. Soaps are generally gentler and less likely to cause irritation.
Other Cleaning Tasks: Tailoring to the Need
For other cleaning tasks, such as cleaning floors, countertops, or other surfaces, the choice depends on the type of soil and the surface material. For general cleaning, both soaps and detergents can be effective. However, for stubborn grease or grime, detergents may offer a more powerful cleaning solution.
Environmental Impact: A Comparative Look
The environmental impact of soaps and detergents is a significant consideration.
Soap's Biodegradability Advantage
Soaps, being naturally derived, generally possess a significant biodegradability advantage over detergents. They decompose readily in the environment, minimizing pollution.
Detergent's Evolving Sustainability
While older detergent formulations posed environmental concerns, modern detergents are increasingly designed with biodegradability in mind. However, the ingredients and manufacturing processes of detergents still have a larger environmental footprint compared to soap. Consider looking for detergents labeled as eco-friendly, biodegradable, and phosphate-free.
Beyond the Basics: Understanding Specific Types
The world of soaps and detergents extends beyond the basic distinctions.
Different Types of Soaps:
- Castile Soap: Made from olive oil, known for its versatility and mildness.
- Sodium-based soaps: Solid soaps, commonly found in bars.
- Potassium-based soaps: Softer soaps, often used in liquid formulations.
Different Types of Detergents:
- Powder Detergents: Effective for general cleaning, often containing builders to enhance cleaning power.
- Liquid Detergents: Convenient for use, effective in both hot and cold water.
- Pods/Packets: Convenient pre-measured doses for easy use.
- Concentrated Detergents: More environmentally friendly due to reduced packaging and shipping weight.
Making Informed Choices for a Cleaner Future
Ultimately, the choice between soap and detergent hinges on individual needs and preferences. Understanding their chemical composition, cleaning mechanisms, and environmental impact allows for informed decisions. For gentle cleaning and environmental consciousness, soap often presents an attractive option. For powerful cleaning and hard water situations, detergents are the more effective choice. By considering the specific cleaning task and prioritizing environmentally friendly options, you can make informed choices that contribute to a cleaner home and a healthier planet. The key takeaway? Both soaps and detergents have their place in the world of cleaning – choosing the right one for the job ensures optimal results.
Latest Posts
Latest Posts
-
What Is The Lcm Of 8 And 10
Mar 12, 2025
-
What Is The Difference Between Serum And Plasma
Mar 12, 2025
-
What Is 35 In Roman Numerals
Mar 12, 2025
-
What Is The Lcm Of 12 8
Mar 12, 2025
-
Lowest Common Multiple Of 8 And 10
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Difference Between A Soap And A Detergent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
