Density Of Water In Si System
Juapaving
Mar 07, 2025 · 6 min read
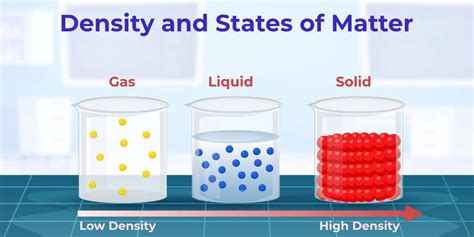
Table of Contents
Density of Water in the SI System: A Comprehensive Guide
The density of water, a seemingly simple concept, plays a crucial role in numerous scientific fields and everyday applications. Understanding its value and the factors influencing it within the International System of Units (SI) is fundamental for accurate calculations and interpretations across various disciplines. This comprehensive guide delves into the intricacies of water density, exploring its variations based on temperature, pressure, and isotopic composition, while also highlighting its significance in diverse scientific and practical contexts.
What is Density?
Density is a fundamental physical property defined as the mass of a substance per unit volume. It essentially tells us how much matter is packed into a given space. The SI unit for density is kilograms per cubic meter (kg/m³), although other units like grams per cubic centimeter (g/cm³) are also commonly used. The formula for density is:
Density (ρ) = Mass (m) / Volume (V)
Understanding density is vital because it allows us to:
- Compare the relative "heaviness" of materials: A higher density means a substance is more compact.
- Predict buoyancy: Objects less dense than water will float.
- Calculate the mass or volume of a substance: Knowing the density and either the mass or volume allows us to calculate the unknown parameter.
- Analyze material properties: Changes in density can indicate changes in the material's composition or structure.
Density of Water: The Standard Value
The density of pure water at its point of maximum density—which is at approximately 4°C (39.2°F) under standard atmospheric pressure—is approximately 999.972 kg/m³. This value is often rounded to 1000 kg/m³ or 1 g/cm³ for simplicity in many calculations. It's crucial to remember that this is an approximation, and the actual density can vary depending on several factors discussed below.
Factors Affecting Water Density
Several factors significantly influence the density of water:
1. Temperature
Temperature is arguably the most significant factor affecting water density. As water is heated from 0°C to 4°C, it becomes denser. This is an unusual property of water, related to its unique hydrogen bonding structure. Above 4°C, water behaves like most other substances, becoming less dense as temperature increases. The density decreases because the increased kinetic energy of the molecules causes them to move further apart. This phenomenon is crucial for aquatic life, as ice floats on water, insulating the water below and preventing it from freezing solid.
2. Pressure
Pressure also affects water density. Increasing pressure forces water molecules closer together, resulting in a higher density. This effect is more pronounced at higher pressures, particularly in deep ocean environments where the immense pressure significantly increases water density. The change in density with pressure is described by the isothermal compressibility of water.
3. Isotopic Composition
Water molecules consist of two hydrogen atoms and one oxygen atom. However, hydrogen has two stable isotopes: protium (¹H) and deuterium (²H), also known as heavy hydrogen. The presence of deuterium affects the density of water. Water enriched in deuterium, known as heavy water (D₂O), has a higher density than normal water (H₂O). The isotopic composition of water varies slightly depending on its source and geographic location, leading to minor variations in its density.
4. Salinity
Dissolved salts and other impurities in water also impact its density. Saltwater, for example, is denser than freshwater because the dissolved salts add mass to the solution without significantly increasing its volume. This difference in density drives ocean currents and plays a crucial role in marine ecosystems.
5. Dissolved Gases
The presence of dissolved gases, such as oxygen and carbon dioxide, slightly affects the density of water. However, this effect is generally less significant compared to temperature, pressure, and salinity.
Applications of Water Density
The density of water is crucial across a vast range of applications:
1. Oceanography and Meteorology
In oceanography, water density is a key parameter for understanding ocean currents, mixing processes, and the stratification of the ocean. Density differences drive thermohaline circulation, a global system of ocean currents that plays a major role in regulating Earth's climate. In meteorology, water density influences the formation of clouds, precipitation, and atmospheric stability.
2. Hydrology and Environmental Science
Water density is essential for understanding hydrological processes, including groundwater flow, river dynamics, and water quality. It's also crucial in environmental science for analyzing water pollution, sediment transport, and the behavior of contaminants in aquatic systems.
3. Engineering and Technology
Water density is critical in engineering applications involving fluid mechanics, hydraulics, and heat transfer. It's used in designing and analyzing various systems, including pipelines, dams, and cooling systems. Accurate density measurements are essential for ensuring the proper functioning and safety of these systems.
4. Chemistry and Biochemistry
In chemistry and biochemistry, water density is vital for preparing solutions, performing titrations, and carrying out various chemical and biological experiments. The density of water is often used as a reference point for determining the density of other substances.
5. Food Science and Industry
In food science, water density is used for determining the quality and consistency of various food products, such as fruit juices, dairy products, and sauces. Density measurements help ensure the proper concentration and quality control of food items.
Measuring Water Density
Several methods are available for measuring the density of water, each with its own level of accuracy and application.
- Hydrometer: A simple and widely used device for measuring the relative density of liquids. It floats in the liquid, and the depth of immersion indicates the density.
- Pycnometer: A precise instrument used for measuring the density of liquids by determining the mass of a known volume of the liquid.
- Digital Density Meter: Modern electronic devices that provide highly accurate density measurements using various principles like oscillation or buoyancy.
- Archimedes' Principle: This principle, based on buoyancy, can be used to determine the density of an object relative to the density of water, offering an indirect method for density determination.
The choice of method depends on the required accuracy, the available resources, and the specific application. High-precision measurements often require specialized equipment and controlled environmental conditions to minimize errors.
Conclusion
The density of water, though seemingly simple, is a complex and dynamic property influenced by various factors including temperature, pressure, and isotopic composition. Understanding these influences and the accurate measurement of water density are crucial across a broad spectrum of scientific disciplines and industrial applications. From understanding ocean currents to designing efficient cooling systems, the knowledge of water density is fundamental to numerous critical processes and technologies. This guide provides a comprehensive overview of this essential physical property, highlighting its significance and diverse applications in various fields. Continued research and advancements in measurement techniques will further enhance our understanding of this crucial aspect of the natural world and its influence on human activities.
Latest Posts
Latest Posts
-
Do Plant Cells Have A Mitochondria
Mar 09, 2025
-
Least Common Multiple Of 5 And 11
Mar 09, 2025
-
What Is The Percentage Of Water In Urine
Mar 09, 2025
-
Which Structure Contains Blood With The Highest Oxygen Concentration
Mar 09, 2025
-
What Is A Property Of A Solid
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about Density Of Water In Si System . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
