What Is A Property Of A Solid
Juapaving
Mar 09, 2025 · 7 min read
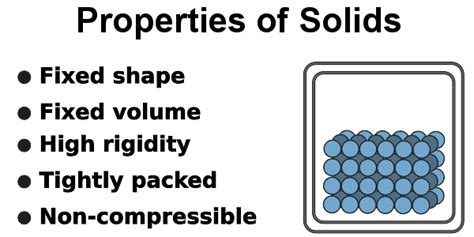
Table of Contents
What is a Property of a Solid? A Deep Dive into the Characteristics of Solids
Solids are one of the four fundamental states of matter, alongside liquids, gases, and plasmas. Understanding the properties of solids is crucial in various fields, from materials science and engineering to chemistry and physics. This comprehensive guide delves into the diverse characteristics that define solids, exploring their structure, behavior, and the forces that govern them.
Defining Solids: A Matter of Structure and Behavior
At its core, a solid is defined by its fixed shape and volume. Unlike liquids and gases, which readily adapt to the shape of their container, solids resist deformation. This rigidity stems from the strong intermolecular forces holding their constituent particles (atoms, ions, or molecules) together in a highly ordered arrangement. This ordered arrangement is a key characteristic that distinguishes solids from other states of matter.
Crystalline vs. Amorphous Solids: A Structural Distinction
Solids can be broadly classified into two main categories based on the arrangement of their constituent particles:
-
Crystalline Solids: These possess a highly ordered, three-dimensional, repeating structure called a crystal lattice. The atoms, ions, or molecules are arranged in a regular, geometric pattern that extends throughout the entire solid. Examples include table salt (NaCl), diamonds (C), and quartz (SiO₂). This regular arrangement leads to distinct properties like anisotropy, where properties vary depending on the direction.
-
Amorphous Solids: Unlike crystalline solids, amorphous solids lack a long-range ordered structure. Their constituent particles are arranged randomly, similar to a liquid, but they retain a fixed shape and volume. Examples include glass, rubber, and many plastics. Their disordered structure often results in isotropy, where properties are consistent in all directions.
Key Physical Properties of Solids
The properties of a solid are determined by several factors, including the type of bonding between its constituent particles, the arrangement of these particles, and the strength of the intermolecular forces. Let's explore some of the most important physical properties:
1. Density: Mass Packed Tightly
Density is a measure of how much mass is packed into a given volume. Solids generally have high densities compared to liquids and gases because their particles are tightly packed together. The density of a solid depends on the mass of its constituent particles and how closely they are packed within the crystal lattice or amorphous structure. High density materials are often strong and durable.
2. Hardness: Resistance to Scratching and Indentation
Hardness measures a solid's resistance to being scratched or indented. It reflects the strength of the bonds between the constituent particles. Materials like diamonds are incredibly hard due to the strong covalent bonds between their carbon atoms. Hardness is crucial in applications requiring wear resistance, such as cutting tools and protective coatings. Hardness testing involves various methods, including the Mohs Hardness Scale, which is a relative scale comparing materials against each other.
3. Melting Point: The Transition to Liquid
The melting point is the temperature at which a solid transforms into a liquid. At the melting point, the kinetic energy of the particles overcomes the intermolecular forces holding them in a fixed structure. The melting point is a characteristic property of a specific solid and can be used for identification. Stronger intermolecular forces result in higher melting points.
4. Boiling Point (Sublimation): The Transition to Gas (or Directly to Gas)
While boiling point is primarily associated with liquids, it's relevant to solids in the context of sublimation. Some solids, like dry ice (solid CO₂), can directly transition from the solid phase to the gaseous phase without melting. This transition is known as sublimation, and the temperature at which it occurs is the solid's sublimation point. Again, stronger intermolecular forces lead to higher sublimation temperatures.
5. Brittleness and Malleability: Response to Stress
-
Brittleness: This property refers to a solid's tendency to fracture or shatter under stress. Brittle materials have limited ability to deform before breaking. Ceramics and glass are examples of brittle materials.
-
Malleability: This is the ability of a solid to be deformed under pressure, such as hammering or rolling, without fracturing. Metals are generally malleable due to the ability of their atoms to slide past each other.
6. Ductility: The Ability to be Drawn into Wires
Ductility is closely related to malleability, but it specifically refers to a solid's ability to be drawn into wires. Metals are often ductile because their atoms can be easily rearranged under tensile stress. This property is crucial for the production of wires and cables.
7. Elasticity: Returning to Original Shape
Elasticity describes a solid's ability to return to its original shape after being deformed by an external force. When the force is removed, the solid springs back to its initial state. This property is essential in applications involving springs, rubber bands, and other flexible materials. The degree of elasticity depends on the strength and nature of the intermolecular forces.
8. Tensile Strength: Resistance to Pulling Forces
Tensile strength measures a solid's resistance to being pulled apart. It is the maximum stress a material can withstand before breaking under tension. High tensile strength is crucial in applications requiring high structural integrity, such as bridges and aircraft.
9. Compressive Strength: Resistance to Squeezing Forces
Compressive strength measures a solid's resistance to being squeezed or compressed. It's the maximum pressure a material can withstand before it fails under compression. This is critical in construction and engineering where materials experience significant compressive loads.
10. Thermal Conductivity: Heat Transfer Efficiency
Thermal conductivity refers to a solid's ability to conduct heat. Materials with high thermal conductivity, like metals, efficiently transfer heat, while materials with low thermal conductivity, like wood, are insulators. This property is vital in applications ranging from heat sinks in electronics to insulation in buildings.
11. Electrical Conductivity: Ability to Conduct Electricity
Electrical conductivity refers to a solid's ability to conduct electricity. Metals are excellent electrical conductors due to the presence of freely moving electrons in their crystal structures. Insulators, on the other hand, have tightly bound electrons and offer high resistance to the flow of electricity. This property is fundamental in electrical wiring, electronics, and many other technologies.
12. Magnetic Properties: Interaction with Magnetic Fields
Solids can exhibit various magnetic properties, including diamagnetism, paramagnetism, ferromagnetism, and antiferromagnetism. These properties arise from the interactions of electrons within the solid's atoms with external magnetic fields. Ferromagnetic materials, like iron, nickel, and cobalt, are strongly attracted to magnets. These properties are crucial for numerous applications including data storage, electric motors, and generators.
Factors Influencing Solid Properties: A Closer Look
The properties of a solid are not independent but are interconnected and influenced by several key factors:
1. Type of Chemical Bonding: The Glue That Holds It Together
The type of chemical bonding significantly impacts a solid's properties. Ionic bonds, found in salts, lead to brittle solids with high melting points. Covalent bonds, like those in diamonds, result in hard, strong solids with very high melting points. Metallic bonds, characteristic of metals, lead to ductile, malleable solids with high electrical and thermal conductivity. Van der Waals forces, relatively weak forces, are responsible for the properties of many molecular solids.
2. Crystal Structure: Order and Arrangement
The arrangement of atoms, ions, or molecules in a crystal lattice profoundly influences a solid's properties. Different crystal structures exhibit different symmetries and packing efficiencies, leading to variations in density, hardness, and other properties. For example, the cubic close-packed structure is denser than the body-centered cubic structure.
3. Impurities and Defects: Imperfections with Impact
The presence of impurities and defects in the crystal structure can significantly alter a solid's properties. Impurities can change a material's strength, conductivity, and other characteristics. Defects, such as vacancies or dislocations, can affect the material's mechanical properties and can sometimes be beneficial, influencing material workability.
4. Temperature and Pressure: External Influences
Temperature and pressure can also affect the properties of solids. Increasing the temperature can lead to expansion, potentially causing changes in density and mechanical properties. High pressure can cause phase transitions, changing the crystal structure and resulting in altered properties.
Conclusion: Understanding Solids, Understanding Matter
The properties of solids are a complex interplay of structure, bonding, and external factors. Understanding these properties is essential for designing and developing new materials with desired characteristics. From the hardness of diamonds to the ductility of copper, the diversity of solid properties reflects the rich variety of atomic arrangements and intermolecular forces in the world around us. Further research and exploration continue to reveal new insights into the fascinating world of solid-state physics and materials science, leading to advancements in technology and a deeper comprehension of the matter that shapes our world.
Latest Posts
Latest Posts
-
What Are The Factors For 88
Mar 09, 2025
-
What Are The Prime Factors Of 225
Mar 09, 2025
-
What Are All The Factors Of 99
Mar 09, 2025
-
Difference Between Monohybrid And Dihybrid Cross
Mar 09, 2025
-
The Capacity To Do Work Is Known As
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about What Is A Property Of A Solid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
