Denaturation Of A Protein Results In
Juapaving
Mar 14, 2025 · 7 min read
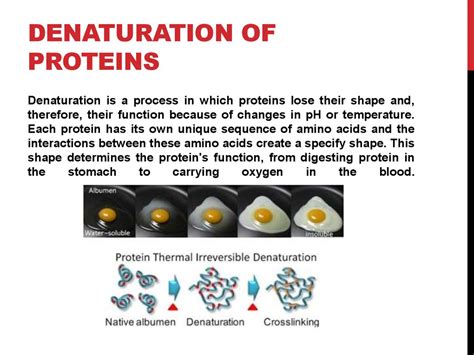
Table of Contents
Denaturation of a Protein Results In: A Comprehensive Guide
Protein denaturation, a process that alters the native structure of a protein, significantly impacts its functionality. Understanding the consequences of denaturation is crucial in various fields, from biochemistry to food science and medicine. This comprehensive guide will delve into the intricacies of protein denaturation, exploring its causes, mechanisms, and wide-ranging effects.
What is Protein Denaturation?
Proteins are biological macromolecules with complex three-dimensional structures essential for their biological functions. These structures are meticulously organized through various levels of organization: primary, secondary, tertiary, and sometimes quaternary structures. The primary structure refers to the linear sequence of amino acids. The secondary structure involves local folding patterns like alpha-helices and beta-sheets, stabilized by hydrogen bonds. The tertiary structure describes the overall three-dimensional arrangement of a polypeptide chain, determined by interactions between amino acid side chains (hydrophobic interactions, disulfide bonds, ionic bonds, hydrogen bonds). Finally, the quaternary structure involves the assembly of multiple polypeptide chains to form a functional protein complex.
Protein denaturation is the disruption of these higher-order structures (secondary, tertiary, and quaternary), leaving only the primary structure intact. This process unfolds the protein, altering its shape and consequently, its biological activity. Importantly, denaturation doesn't break the peptide bonds within the protein's primary structure. The amino acid sequence remains the same; however, the protein loses its native conformation, rendering it non-functional.
Causes of Protein Denaturation
Several factors can trigger protein denaturation. Understanding these factors is crucial in preventing denaturation in applications where preserving protein structure is vital. The most common causes include:
1. Heat
Heat denaturation is perhaps the most well-known cause. Elevated temperatures provide sufficient energy to overcome the weak interactions (hydrogen bonds, hydrophobic interactions) that stabilize the protein's higher-order structures. This leads to unfolding and loss of function. This is evident in cooking eggs, where the transparent egg white (albumin) turns opaque upon heating due to protein denaturation.
2. pH Changes
Extreme pH values (both highly acidic and highly alkaline) can disrupt the electrostatic interactions and hydrogen bonds crucial for maintaining protein structure. Changes in pH alter the charge distribution on amino acid side chains, disrupting ionic bonds and affecting the overall protein folding.
3. Organic Solvents
Organic solvents, such as ethanol and acetone, can interfere with hydrophobic interactions within a protein, leading to denaturation. These solvents disrupt the hydrophobic core of the protein, causing unfolding.
4. Chaotropic Agents
Chaotropic agents are substances that disrupt the structure of water molecules, thereby weakening the hydrophobic interactions that contribute to protein folding. Urea and guanidinium chloride are examples of commonly used chaotropic agents. These agents increase the solubility of nonpolar substances in water, disrupting the hydrophobic interactions that stabilize the protein's structure.
5. Detergents
Detergents, like sodium dodecyl sulfate (SDS), are amphipathic molecules with both hydrophilic and hydrophobic regions. They interact with the hydrophobic regions of proteins, disrupting hydrophobic interactions and causing denaturation. SDS is frequently used in laboratory techniques like SDS-PAGE to separate proteins based on their size.
6. Mechanical Stress
Physical forces, such as agitation, shearing, and pressure, can also cause protein denaturation. These forces disrupt the delicate balance of interactions within the protein, leading to unfolding. This is particularly relevant in processes like homogenization or intense mixing.
7. Heavy Metal Ions
Heavy metal ions (e.g., mercury, lead) can interact with amino acid side chains, particularly cysteine residues, forming strong bonds and altering the protein's structure. This interaction can disrupt the normal folding pattern and lead to denaturation.
Mechanisms of Protein Denaturation
The mechanisms underlying protein denaturation are complex and depend on the denaturing agent and the protein itself. However, several key steps are often involved:
-
Initial disruption of weak interactions: The denaturing agent initially targets the weaker interactions (hydrogen bonds, hydrophobic interactions, ionic bonds) that maintain the secondary, tertiary, and quaternary structures.
-
Unfolding of the protein: As these weak interactions are disrupted, the protein begins to unfold, losing its organized structure. This unfolding can be partial or complete, depending on the severity of the denaturing conditions.
-
Exposure of hydrophobic residues: The unfolding exposes hydrophobic amino acid side chains that were previously buried within the protein's core. These exposed hydrophobic residues can then interact with other hydrophobic regions or the surrounding environment.
-
Aggregation or precipitation: In some cases, the exposed hydrophobic residues can lead to aggregation, where denatured protein molecules stick together, forming large insoluble clumps. This aggregation can be irreversible.
Consequences of Protein Denaturation
The denaturation of a protein almost always leads to a loss of function. The specific consequences depend on the protein and the extent of denaturation. However, some common consequences include:
1. Loss of Biological Activity:
This is the most significant consequence. Since the protein's three-dimensional structure is directly related to its function (e.g., enzyme activity, receptor binding, structural support), disrupting this structure abolishes its ability to perform its biological role.
2. Altered Solubility:
Denaturation can alter the protein's solubility. Some proteins become less soluble and precipitate out of solution, while others may become more soluble. This change in solubility is often related to the exposure of hydrophobic residues.
3. Increased Susceptibility to Proteolysis:
Denatured proteins are more susceptible to degradation by proteases (enzymes that break down proteins). This is because the exposed peptide bonds are more accessible to the proteases.
4. Changes in Immunogenicity:
Denaturation can alter the protein's antigenic properties, affecting its ability to trigger an immune response. This is relevant in vaccine development and immunodiagnostics.
5. Changes in Physical Properties:
Denaturation often results in changes in the protein's physical properties, such as viscosity, optical properties (e.g., absorbance at specific wavelengths), and electrophoretic mobility.
Examples of Denaturation in Everyday Life and Scientific Applications
Protein denaturation is a ubiquitous process with implications across various fields.
1. Food Science:
Cooking meat involves heat denaturation of proteins, making it more digestible and altering its texture. The hardening of an egg white when boiled is another classic example. Pasteurization, a process that uses heat to kill harmful microorganisms in food, relies on the heat denaturation of microbial proteins.
2. Medicine:
Protein denaturation plays a crucial role in various medical applications. Sterilization techniques often rely on denaturing proteins in microorganisms. Certain medical treatments involve carefully controlled denaturation to modify protein properties or eliminate unwanted protein activity.
3. Biochemistry and Molecular Biology:
Techniques like SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) utilize protein denaturation to separate proteins based on their size. Researchers often denature proteins to study their primary structure or to analyze their individual components.
4. Industrial Applications:
Several industrial processes involve protein denaturation. Leather tanning utilizes chemical treatments that denature collagen proteins in animal hides, increasing their strength and durability.
Reversible vs. Irreversible Denaturation
While denaturation often implies irreversible changes, some proteins can refold into their native structure under appropriate conditions. This is known as reversible denaturation. The reversibility depends on several factors, including the nature of the denaturing agent, the extent of denaturation, and the protein itself. If the denaturing conditions are mild and the protein's primary structure remains intact, it might be able to spontaneously refold into its native state upon removal of the denaturing agent. However, if the denaturation is severe, involving extensive unfolding and aggregation, the process is usually irreversible. This irreversible denaturation often leads to the formation of stable aggregates, which are difficult to refold.
Conclusion
Protein denaturation is a complex process with far-reaching consequences. Understanding the causes, mechanisms, and effects of denaturation is essential in various disciplines. From cooking to medical treatments and industrial processes, the control and manipulation of protein denaturation are critical for optimizing outcomes and preserving protein functionality. The ability to predict and control protein denaturation is crucial for ensuring the stability and functionality of proteins in numerous applications. Further research into the intricacies of protein folding and unfolding continues to expand our understanding of this vital biological process. This knowledge not only aids our understanding of natural biological systems but also helps in the design and development of novel biomaterials and therapeutic strategies.
Latest Posts
Latest Posts
-
Is Melting Ice Chemical Or Physical Change
Mar 14, 2025
-
How Many Valence Electrons Are In Strontium
Mar 14, 2025
-
Is Gold A Mixture Or Pure Substance
Mar 14, 2025
-
Cellulose Is An Example Of A
Mar 14, 2025
-
How Many Lines Of Symmetry Square
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Denaturation Of A Protein Results In . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
