Can A Pure Substance Be Separated
Juapaving
Mar 19, 2025 · 6 min read
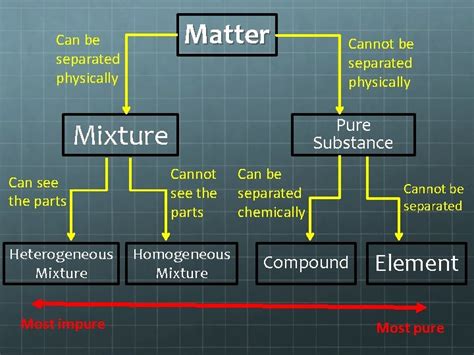
Table of Contents
Can a Pure Substance Be Separated? Exploring the Nature of Matter
The question of whether a pure substance can be separated is a fundamental one in chemistry, touching upon the very definition of purity and the nature of matter itself. The short answer is: no, a truly pure substance cannot be separated into different components by physical means. However, the nuanced answer delves into the complexities of what constitutes "pure," the limits of our separation techniques, and the potential for chemical changes to alter the substance.
Defining Purity and Substances
Before we delve into the separation question, it's crucial to define our terms. A pure substance is a form of matter that has a constant chemical composition and distinct chemical properties. This means it's made up of only one type of atom or molecule. Examples include elements like gold (Au) or oxygen (O₂), and compounds like water (H₂O) or table salt (NaCl).
Conversely, a mixture is a combination of two or more pure substances that are physically combined but not chemically bonded. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water). Crucially, mixtures can be separated into their constituent components by physical methods.
The key difference lies in the level of interaction between the components. In a pure substance, the atoms or molecules are bound together by strong chemical bonds, requiring chemical reactions to break them apart. In a mixture, the components are held together by weaker forces (like van der Waals forces or hydrogen bonds), allowing separation through physical means.
Physical Methods of Separation: Effective for Mixtures, Ineffective for Pure Substances
A wide array of physical methods exist for separating mixtures. The choice of method depends on the properties of the components, such as their boiling points, melting points, solubility, density, and particle size. Here are some examples:
1. Filtration: Separating Solids from Liquids
Filtration uses a porous material (like filter paper) to separate a solid from a liquid. The liquid passes through the filter, leaving the solid behind. This is effective for separating sand from water, for instance.
2. Distillation: Separating Liquids with Different Boiling Points
Distillation exploits the difference in boiling points of liquids. The mixture is heated, and the component with the lower boiling point vaporizes first, is condensed, and collected separately. This is how we obtain purified water from seawater.
3. Evaporation: Separating Dissolved Solids from Liquids
Evaporation involves heating a solution until the liquid evaporates, leaving behind the dissolved solid. This technique is commonly used to obtain salt from seawater.
4. Chromatography: Separating Components Based on Differential Adsorption
Chromatography utilizes the different affinities of components for a stationary and mobile phase to separate them. The mixture is passed through a column or spread across a surface, and the components separate based on their varying interactions with the phases. This technique is widely used in analytical chemistry for separating complex mixtures.
5. Crystallization: Separating Solids Based on Solubility
Crystallization relies on the difference in solubility of components at different temperatures. A saturated solution is cooled, causing the less soluble component to crystallize out of the solution. This method is used to purify substances and obtain large, well-formed crystals.
6. Centrifugation: Separating Components Based on Density
Centrifugation uses centrifugal force to separate components of different densities. The denser components settle to the bottom of the tube while the lighter ones remain at the top. This is often used to separate blood cells from plasma.
The Limits of Physical Separation: Purity and Impurities
While these physical methods are highly effective for separating mixtures, they are ineffective for separating a truly pure substance. This is because a pure substance, by definition, consists of only one type of atom or molecule. There's nothing to separate! Any attempt to "separate" a pure substance using physical methods will either leave the substance unchanged or potentially lead to its decomposition, which is a chemical, not a physical change.
However, it's important to consider the practical limitations of what we consider "pure." Even substances considered "pure" in everyday life contain trace amounts of impurities. These impurities may be present in very small quantities, perhaps due to incomplete synthesis or contamination during handling. These trace impurities can sometimes be separated using advanced physical techniques, but this does not mean the original substance was a mixture; rather, it was an impure sample of a pure substance. The removal of these impurities through advanced techniques brings the sample closer to true purity.
Chemical Methods: Breaking Bonds and Creating New Substances
To break apart a pure substance into its constituent elements or simpler compounds, you need to employ chemical methods. Chemical reactions involve the breaking and formation of chemical bonds, transforming the substance into new ones. For example, the electrolysis of water uses an electric current to break the water molecules (H₂O) into hydrogen (H₂) and oxygen (O₂) gases. This is a chemical change, not a physical separation.
Isotopes and the Nuances of Purity
The concept of purity becomes even more nuanced when considering isotopes. Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. For example, hydrogen has three isotopes: protium, deuterium, and tritium. While all three are hydrogen, they have different masses. Separating isotopes is extremely challenging and typically requires sophisticated techniques like isotopic separation using mass spectrometry or gas diffusion. However, separating isotopes doesn't break apart the substance; it simply separates different versions of the same element.
Practical Applications and Implications
The ability (or inability) to separate a pure substance has significant implications across various fields:
- Material Science: Understanding the purity of materials is crucial for determining their properties and applications. High-purity materials are essential in semiconductor manufacturing, aerospace engineering, and biomedical applications.
- Analytical Chemistry: The separation of mixtures is central to analytical chemistry. Techniques like chromatography and electrophoresis allow scientists to identify and quantify the components of complex mixtures.
- Pharmaceutical Industry: The purity of drugs and pharmaceutical ingredients is critical for safety and efficacy. Rigorous purification methods are used to remove impurities and ensure product quality.
- Environmental Science: Separating pollutants from environmental samples is vital for monitoring and remediation efforts. Techniques like filtration and extraction are used to isolate and analyze contaminants.
Conclusion: Purity, Separation, and the Ongoing Pursuit of Precision
The question of whether a pure substance can be separated is complex but ultimately straightforward. A truly pure substance, composed of only one type of atom or molecule held together by strong chemical bonds, cannot be separated into different components by physical methods. Any attempt to do so will either leave the substance unchanged or lead to a chemical transformation. However, the practical notion of purity is often limited by trace impurities. Removing these impurities brings a substance closer to true purity, using a range of sophisticated techniques. Understanding the nuances of purity and the limitations of separation methods is crucial across numerous scientific and industrial disciplines. The ongoing pursuit of greater purity in materials and substances drives advancements in separation technology and chemical analysis, furthering our understanding of matter and its properties.
Latest Posts
Latest Posts
-
What Is The Conservation Of Charge
Mar 19, 2025
-
How Many Faces On A Dice
Mar 19, 2025
-
Which Kingdom Does Not Contain Any Eukaryotes
Mar 19, 2025
-
Database Is A Collection Of Related Data
Mar 19, 2025
-
Is Distilled Water An Acid Or A Base
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Can A Pure Substance Be Separated . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
