Is Distilled Water An Acid Or A Base
Juapaving
Mar 19, 2025 · 5 min read
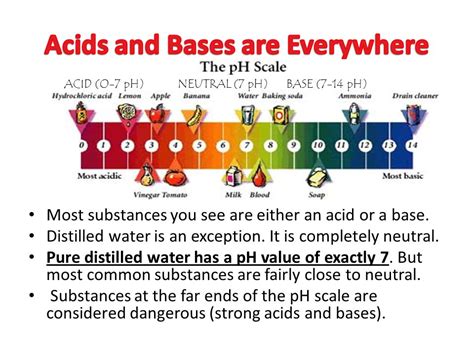
Table of Contents
Is Distilled Water an Acid or a Base? Understanding pH and Purity
Distilled water, often lauded for its purity, sparks a common question: is it acidic or basic? The answer, while seemingly simple, delves into the intricacies of pH, water's self-ionization, and the impact of impurities. This comprehensive guide explores these aspects, dispelling myths and providing a clear understanding of distilled water's nature.
Understanding pH: The Acid-Base Scale
Before we classify distilled water, let's revisit the fundamental concept of pH. The pH scale, ranging from 0 to 14, measures the concentration of hydrogen ions (H⁺) in a solution. A pH of 7 represents neutrality, where the concentration of H⁺ ions equals the concentration of hydroxide ions (OH⁻). Solutions with a pH below 7 are acidic, indicating a higher concentration of H⁺ ions. Conversely, solutions with a pH above 7 are basic (or alkaline), signifying a higher concentration of OH⁻ ions.
Key takeaway: The pH scale is logarithmic, meaning each whole number change represents a tenfold difference in H⁺ ion concentration. A pH of 6 is ten times more acidic than a pH of 7, while a pH of 5 is a hundred times more acidic.
The Self-Ionization of Water: A Delicate Balance
Pure water, even distilled water, doesn't exist as simply H₂O molecules. A small fraction of water molecules undergo self-ionization, a process where one water molecule donates a proton (H⁺) to another, forming a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻). This reaction is represented as:
2H₂O ⇌ H₃O⁺ + OH⁻
This equilibrium reaction is crucial. In pure water, the concentration of H₃O⁺ ions equals the concentration of OH⁻ ions, maintaining a neutral pH of 7 at 25°C (77°F). This equilibrium is very sensitive to temperature and the presence of impurities.
Factors Affecting pH of Water
Several factors can influence the pH of seemingly pure water, including:
-
Temperature: As temperature increases, the rate of self-ionization increases, slightly shifting the equilibrium and leading to a marginally lower pH (though still close to neutral). This is because the equilibrium constant for water's self-ionization is temperature-dependent.
-
Dissolved Gases: Carbon dioxide (CO₂) from the atmosphere readily dissolves in water, forming carbonic acid (H₂CO₃), a weak acid. This dissolved CO₂ can slightly lower the pH of water, making it mildly acidic.
-
Dissolved Minerals: Even trace amounts of dissolved minerals, like calcium, magnesium, or sodium, from the source water or the container can impact the pH. These minerals can act as buffers, resisting changes in pH or even shifting the balance toward alkalinity.
-
Contamination during Distillation: The distillation process aims to remove impurities, but traces of contaminants can still remain if the process isn't perfect. This residual contamination can influence the pH.
Is Distilled Water Truly Neutral?
Ideally, distilled water should have a pH of 7, signifying neutrality. However, in reality, it's difficult to achieve perfect neutrality due to the factors mentioned above. Many commercially available distilled waters have a slightly acidic pH, typically ranging from 5.8 to 7.0. This slight acidity is primarily due to dissolved CO₂ from the atmosphere. The lower pH is usually not a concern for most applications.
Important Note: A slightly acidic pH doesn't automatically imply contamination. It often reflects the subtle equilibrium dynamics and the presence of dissolved atmospheric gases.
Applications of Distilled Water: Where Purity Matters
Distilled water's purity makes it suitable for various applications where impurities can interfere with processes or products. Here are some examples:
-
Laboratory Work: In scientific research, distilled water ensures that experiments aren't skewed by the presence of ions or other dissolved substances.
-
Automotive Batteries: Using distilled water to top up lead-acid batteries prevents the accumulation of impurities that can degrade performance.
-
Medical Applications: Distilled water is often used in pharmaceutical preparations and medical equipment where purity is critical to avoid contamination.
-
Ironing: Some individuals prefer using distilled water in steam irons to avoid mineral buildup and prolong the appliance's lifespan.
Differentiating Distilled Water from other Purified Waters
It's essential to differentiate distilled water from other types of purified water like deionized water and reverse osmosis water. While all aim for high purity, their processes differ.
-
Deionized water: This water undergoes deionization, a process that removes ions using ion-exchange resins. While exceptionally pure regarding ions, it may still contain non-ionic impurities.
-
Reverse osmosis water: This water is purified by forcing it through a semipermeable membrane, filtering out many impurities. Like deionized water, it can contain some non-ionic contaminants.
Conclusion: Addressing the Question
In essence, distilled water is ideally neutral (pH 7) but often exhibits a slightly acidic pH due to dissolved carbon dioxide from the atmosphere and the inherent self-ionization of water. This slight acidity is generally inconsequential for most uses. The purity of distilled water makes it invaluable in various applications where the absence of impurities is paramount. However, it's crucial to remember that even distilled water isn't entirely free from all substances and its pH can fluctuate due to environmental conditions. Understanding the factors influencing its pH helps to correctly interpret its properties and use it effectively in various applications. The term "acidic" or "basic" in the context of distilled water should be considered relative, and the small variations around pH 7 should not be cause for concern in most situations.
Latest Posts
Latest Posts
-
What Is Study Of Flowers Called
Mar 19, 2025
-
What Is The Complement Of A 45 Degree Angle
Mar 19, 2025
-
What Is The Secondary Structure Of Dna
Mar 19, 2025
-
What Is The Multiples Of 40
Mar 19, 2025
-
Least Common Multiple Of 60 And 24
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Distilled Water An Acid Or A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
