Calculate The Molar Mass Of Calcium Nitrate
Juapaving
Mar 19, 2025 · 5 min read
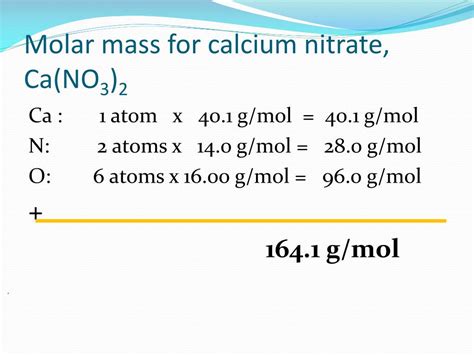
Table of Contents
Calculating the Molar Mass of Calcium Nitrate: A Comprehensive Guide
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This guide will delve into the process of calculating the molar mass of calcium nitrate (Ca(NO₃)₂), explaining each step in detail and highlighting important considerations. We'll also explore the broader applications of molar mass calculations and offer tips for accuracy.
Understanding Molar Mass
Before we embark on the calculation, let's clarify what molar mass represents. Molar mass is the mass of one mole of a substance. A mole is a unit in chemistry representing Avogadro's number (approximately 6.022 x 10²³) of particles, whether they are atoms, molecules, ions, or other specified entities. Essentially, the molar mass tells us the mass of 6.022 x 10²³ molecules (or formula units in the case of ionic compounds like calcium nitrate) of a given substance. It is typically expressed in grams per mole (g/mol).
The Composition of Calcium Nitrate
To calculate the molar mass of calcium nitrate, Ca(NO₃)₂, we must first understand its chemical composition. This ionic compound consists of:
- One calcium ion (Ca²⁺): Calcium is an alkaline earth metal found in Group 2 of the periodic table.
- Two nitrate ions (NO₃⁻): The nitrate ion is a polyatomic anion composed of one nitrogen atom and three oxygen atoms.
This formula indicates that one formula unit of calcium nitrate contains one calcium ion and two nitrate ions. This ratio is critical for our molar mass calculation.
Calculating the Molar Mass of Ca(NO₃)₂: A Step-by-Step Approach
The calculation involves summing the atomic masses of all atoms present in one formula unit of calcium nitrate, taking into account the number of atoms of each element. We'll use the standard atomic masses from the periodic table. Remember to always refer to the most up-to-date periodic table for the most accurate atomic mass values.
Step 1: Determine the Atomic Masses
From a periodic table, we find the atomic masses (approximately):
- Calcium (Ca): 40.08 g/mol
- Nitrogen (N): 14.01 g/mol
- Oxygen (O): 16.00 g/mol
Step 2: Calculate the Mass Contribution of Each Element
- Calcium (Ca): 1 Ca atom x 40.08 g/mol = 40.08 g/mol
- Nitrogen (N): 2 N atoms x 14.01 g/mol = 28.02 g/mol (Note: There are two nitrogen atoms in each nitrate ion, and two nitrate ions in the formula unit.)
- Oxygen (O): 6 O atoms x 16.00 g/mol = 96.00 g/mol (Note: There are three oxygen atoms in each nitrate ion, and two nitrate ions in the formula unit, resulting in six oxygen atoms total.)
Step 3: Sum the Mass Contributions
To obtain the molar mass of calcium nitrate, we add the mass contributions of each element:
40.08 g/mol (Ca) + 28.02 g/mol (N) + 96.00 g/mol (O) = 164.10 g/mol
Therefore, the molar mass of calcium nitrate, Ca(NO₃)₂, is approximately 164.10 g/mol.
Significance and Applications of Molar Mass
The molar mass of calcium nitrate, and molar masses in general, are vital for numerous chemical calculations and applications, including:
-
Stoichiometry: Molar mass is fundamental to stoichiometric calculations, allowing us to convert between mass and moles of a substance. This is crucial for determining reactant quantities, product yields, and limiting reagents in chemical reactions. For example, knowing the molar mass allows us to determine how many grams of calcium nitrate are needed to react completely with a specific amount of another reactant.
-
Solution Preparation: Molar mass is essential when preparing solutions of known concentrations (e.g., molarity). To prepare a solution of a specific molarity, we need to know the molar mass to accurately weigh out the required amount of solute.
-
Titrations: In titrations, where a solution of known concentration is used to determine the concentration of an unknown solution, molar mass is crucial for interpreting the results.
-
Gas Law Calculations: The ideal gas law (PV=nRT) utilizes the number of moles (n) of a gas, which is directly related to its mass and molar mass.
-
Determining Empirical and Molecular Formulas: Knowing the molar mass of a compound can help determine its molecular formula, given its empirical formula (the simplest whole number ratio of atoms).
-
Understanding Chemical Properties: The molar mass provides insight into the mass relationships within a substance and can contribute to understanding its chemical behavior and reactivity.
Ensuring Accuracy in Molar Mass Calculations
Accuracy is paramount when calculating molar masses. Here are some tips to ensure precision:
-
Use an Up-to-Date Periodic Table: Always use a reliable and current periodic table, as atomic masses may have slight revisions.
-
Pay Attention to Significant Figures: Maintain the appropriate number of significant figures throughout the calculation. The final answer should reflect the precision of the least precise measurement used.
-
Double-Check Your Work: Carefully review each step of the calculation to avoid errors in arithmetic or unit conversions.
-
Use Consistent Units: Maintain consistent units throughout the calculation (e.g., grams per mole).
-
Understand the Chemical Formula: Correctly interpreting the chemical formula is crucial to ensure the right number of atoms of each element is included in the calculation.
Beyond Calcium Nitrate: Calculating Molar Mass for Other Compounds
The method described for calcium nitrate is applicable to calculating the molar mass of any compound. Simply identify the elements present, determine their atomic masses, account for the number of atoms of each element in the molecule or formula unit, and sum the individual atomic masses. This principle applies to both simple and complex molecules, including organic compounds and those containing polyatomic ions.
Conclusion
Calculating the molar mass of calcium nitrate, or any compound, is a fundamental skill in chemistry. Understanding the underlying principles and following the steps carefully will ensure accurate results. The ability to perform these calculations is essential for mastering various chemical concepts and performing numerous chemical analyses. By employing the strategies outlined in this guide and paying attention to detail, one can confidently approach any molar mass calculation with precision and accuracy.
Latest Posts
Latest Posts
-
How To Make Ratio Into Percent
Mar 19, 2025
-
What Unit Is Used To Measure Bacteria
Mar 19, 2025
-
Why Are Noble Gases Not Reactive
Mar 19, 2025
-
What Step Of Aerobic Respiration Generates The Most Atp
Mar 19, 2025
-
What Is The Least Common Multiple Of 10 And 25
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Molar Mass Of Calcium Nitrate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
