Why Are Noble Gases Not Reactive
Juapaving
Mar 19, 2025 · 6 min read
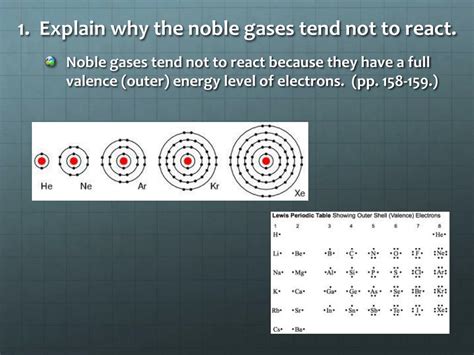
Table of Contents
Why Are Noble Gases Not Reactive? Unraveling the Mystery of Inertness
The noble gases, also known as inert gases, occupy Group 18 of the periodic table. Their unique characteristic, and the reason for their name, is their extreme lack of reactivity. Understanding why noble gases are so unreactive requires delving into the fascinating world of atomic structure and electron configuration. This article will explore the fundamental reasons behind their inertness, examining their electronic structure, ionization energies, and the role of quantum mechanics in shaping their chemical behavior.
The Key to Inertness: Stable Electron Configurations
The key to understanding the non-reactive nature of noble gases lies in their electron configurations. Each noble gas atom, except helium (He), possesses a full outer electron shell, also known as a valence shell, with eight electrons. This arrangement is exceptionally stable and energetically favorable. Helium, with only two electrons, has a full valence shell because its first electron shell can only hold a maximum of two electrons.
This stable electron configuration is often referred to as an octet. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons, mimicking the stable electron configuration of noble gases. This drive towards stability is the fundamental force behind chemical bonding. Since noble gases already possess this stable configuration, they have little inclination to participate in chemical reactions that would disrupt this arrangement.
Helium's Unique Case: The Duet Rule
While the octet rule applies to most atoms, helium is an exception. Its small size allows its valence shell to be filled with only two electrons, fulfilling what's called the duet rule. This full valence shell provides helium with the same exceptional stability as the other noble gases, explaining its inertness.
High Ionization Energies: Resisting Electron Loss
Another crucial factor contributing to the non-reactivity of noble gases is their exceptionally high ionization energies. Ionization energy is the energy required to remove an electron from a neutral atom. Because noble gases have a stable electron configuration, removing an electron requires a significant amount of energy. This high energy requirement makes it highly improbable that an electron will be lost during a chemical reaction.
The strong electrostatic attraction between the positively charged nucleus and the negatively charged electrons in the filled valence shell further contributes to the high ionization energy. This strong attraction makes it energetically unfavorable for a noble gas atom to lose an electron, further reinforcing their inert nature.
Electron Affinity: The Unwillingness to Gain Electrons
Similarly, noble gases exhibit very low electron affinities. Electron affinity is the energy change that occurs when an electron is added to a neutral atom. Adding an electron to a noble gas atom would disrupt its stable electron configuration, resulting in an energetically unfavorable state. The already complete valence shell repels the additional electron, making it highly unlikely for a noble gas atom to gain an electron in a chemical reaction.
The Role of Quantum Mechanics: Understanding Electron Shells and Orbitals
The stability of noble gas electron configurations isn't just a matter of simple counting; it's rooted in the principles of quantum mechanics. Electrons don't simply orbit the nucleus like planets around a star; instead, they occupy specific atomic orbitals characterized by their energy levels and shapes.
Each electron shell consists of several subshells (s, p, d, f), each capable of holding a specific number of electrons. The full outer shell of noble gases represents a completely filled set of atomic orbitals, leading to a state of minimum energy. This minimal energy state is fundamentally stable, making the noble gases exceptionally unreactive.
Shielding Effect and Effective Nuclear Charge: Protecting the Valence Electrons
The shielding effect also plays a significant role in the stability of noble gases. Inner electrons shield the outer valence electrons from the full attractive force of the positively charged nucleus. However, in noble gases, the shielding is particularly effective due to the filled inner shells. This results in a relatively low effective nuclear charge experienced by the valence electrons, further strengthening the stability of the filled valence shell.
Exceptions to the Rule: Compounds of Noble Gases
While noble gases are largely unreactive, it's crucial to acknowledge that there are exceptions. Under extreme conditions, some noble gases, particularly xenon (Xe), krypton (Kr), and radon (Rn), have been found to form compounds. These compounds are typically formed under high pressure, low temperature, or in the presence of highly reactive species such as fluorine.
These exceptions highlight the fact that even the most stable systems can be manipulated under extreme conditions, overriding the usual energetic preference for stability. However, the formation of noble gas compounds remains exceptionally rare, underscoring the general rule of their inertness.
Xenon Compounds: The Most Notable Exceptions
Xenon, being the largest and least electronegative of the readily available noble gases, is the most likely to form compounds. The relatively large size of the xenon atom allows its valence electrons to be slightly more accessible to other atoms. Compounds such as xenon tetrafluoride (XeF₄) and xenon hexafluoride (XeF₆) have been synthesized, demonstrating the possibility of noble gas reactivity under specific conditions. These compounds, however, are still relatively unstable and require specialized conditions for their formation and maintenance.
Applications of Noble Gases: Leveraging Their Inertness
The extreme inertness of noble gases is not a drawback; it's the basis for many of their valuable applications. Their lack of reactivity makes them ideal for various applications where preventing chemical reactions is crucial.
Lighting and Welding: Creating Inert Atmospheres
Noble gases are frequently used in lighting applications such as neon signs and fluorescent lamps. Their inertness prevents reactions with other components of the lamps, ensuring their longevity and preventing the creation of unwanted byproducts. Similarly, noble gases like argon are employed in welding to create an inert atmosphere around the weld, preventing oxidation and improving the quality of the weld.
Medicine and Scientific Research: Utilizing Inert Properties
Helium, due to its low density and inertness, is used in medical applications such as MRI machines and as a breathable gas in certain medical procedures. Argon, another inert gas, finds applications in various scientific research settings, providing an inert atmosphere for sensitive chemical reactions and preventing unwanted contamination.
Conclusion: A Deep Dive into Inertness
The non-reactive nature of noble gases is a consequence of their stable electron configurations, high ionization energies, low electron affinities, and the principles of quantum mechanics governing their atomic structure. While a few exceptions exist under extreme conditions, their overall inertness makes them incredibly useful in various applications where preventing unwanted chemical reactions is essential. Understanding the reasons behind their inertness allows us to appreciate the intricate interplay of fundamental forces that govern the behavior of matter at the atomic level. The fascinating world of noble gases underscores the importance of electron configuration and quantum mechanics in defining the chemical properties of elements. Further research continues to push the boundaries of our understanding, potentially revealing even more subtle nuances about their seemingly simple, yet profound, inertness.
Latest Posts
Latest Posts
-
Nucleic Acids Are Polymers Of Blank
Mar 19, 2025
-
Ias Exam Qualification And Age Limit
Mar 19, 2025
-
Simplify The Square Root Of 243
Mar 19, 2025
-
What Is The Net Gain Of Atp During Glycolysis
Mar 19, 2025
-
Which Of The Following Are Examples Of Passive Transport
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Why Are Noble Gases Not Reactive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
