Boiling Point Of Water In Kelvin
Juapaving
Mar 10, 2025 · 5 min read
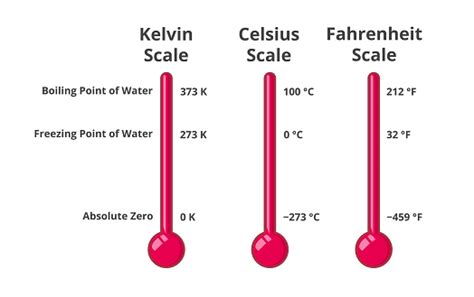
Table of Contents
Boiling Point of Water in Kelvin: A Deep Dive
The boiling point of water, a seemingly simple concept, holds a significant place in various scientific disciplines and everyday life. Understanding its value, particularly in Kelvin, opens doors to a deeper appreciation of thermodynamics, phase transitions, and the behavior of matter. This article delves into the intricacies of water's boiling point in Kelvin, exploring its underlying physics, influencing factors, and practical applications.
What is the Boiling Point of Water in Kelvin?
The boiling point of water at standard atmospheric pressure (101.325 kPa or 1 atm) is 373.15 Kelvin (K). This is equivalent to 100 degrees Celsius (°C) or 212 degrees Fahrenheit (°F). It's crucial to understand that this value is specific to standard conditions. Changes in pressure dramatically affect the boiling point.
Understanding the Kelvin Scale
The Kelvin scale, an absolute thermodynamic temperature scale, is a cornerstone of scientific measurements. Unlike Celsius and Fahrenheit, which use arbitrary zero points, Kelvin's zero point (0 K) represents absolute zero, the theoretical temperature at which all molecular motion ceases. This makes Kelvin particularly useful in scientific calculations and understanding thermal phenomena. The conversion between Kelvin and Celsius is straightforward: K = °C + 273.15.
The Science Behind Boiling: A Molecular Perspective
The boiling point isn't just a number; it's a reflection of the intense molecular activity within water. Water molecules (H₂O) are held together by hydrogen bonds – relatively strong intermolecular forces. These bonds dictate many of water's unique properties, including its relatively high boiling point compared to other molecules of similar size.
Energy and Phase Transitions
At lower temperatures, these hydrogen bonds keep the water molecules relatively close together in a liquid state. As heat is added, the molecules gain kinetic energy, vibrating and moving more rapidly. When the temperature reaches the boiling point, the molecules gain enough energy to overcome the intermolecular forces holding them in the liquid phase. This leads to a phase transition – the transformation from liquid water to gaseous water vapor (steam).
Vapor Pressure and Boiling
Boiling occurs when the vapor pressure of the liquid equals the surrounding atmospheric pressure. Vapor pressure is the pressure exerted by the gaseous molecules above the liquid. As temperature increases, more molecules escape into the gaseous phase, increasing the vapor pressure. When this vapor pressure matches the external pressure, bubbles of vapor can form within the liquid, leading to boiling.
Factors Affecting the Boiling Point of Water
While 373.15 K is the standard boiling point, several factors can influence this value:
Pressure: The Dominant Factor
Pressure is the most significant factor affecting the boiling point. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. This is because the vapor pressure needs to reach a lower value to equal the ambient pressure. Conversely, at higher pressures, the boiling point increases. This principle is exploited in pressure cookers, where higher pressure allows water to reach temperatures above 100°C, leading to faster cooking.
Impurities: A Subtle Influence
The presence of dissolved impurities in water can slightly elevate the boiling point. This phenomenon, known as boiling point elevation, is a colligative property, meaning it depends on the concentration of solute particles, not their identity. The effect is usually small unless a significant amount of solute is present.
Isotopic Composition: A Minor Effect
Water molecules are composed of hydrogen and oxygen isotopes. The isotopic composition can subtly affect the boiling point. For instance, water containing heavier isotopes of hydrogen (deuterium) or oxygen will have a slightly higher boiling point. However, this effect is generally negligible for most practical purposes.
Applications of Water's Boiling Point
The boiling point of water is crucial in a vast array of applications, from everyday cooking to sophisticated industrial processes:
Cooking and Food Preparation
The boiling point of water is fundamental to many cooking methods. Boiling water is used for cooking pasta, vegetables, and eggs. The precise temperature ensures even cooking and prevents burning.
Sterilization and Sanitation
Boiling water is an effective method for sterilizing utensils and equipment, killing bacteria and other microorganisms. This is particularly important in healthcare and food processing.
Industrial Processes
Many industrial processes rely on the boiling point of water. Steam generation for power plants, cooling systems, and various chemical reactions all utilize the phase transition of water.
Scientific Research
In scientific laboratories, the boiling point of water serves as a reference point for calibrating temperature sensors and conducting experiments involving temperature-sensitive reactions.
Measuring the Boiling Point of Water
Accurately measuring the boiling point requires careful consideration of the experimental setup and conditions:
The Importance of Controlled Conditions
Precise measurements demand controlled conditions, primarily maintaining constant atmospheric pressure. Variations in pressure will significantly affect the boiling point.
Thermometers and Calibration
High-quality thermometers calibrated against known standards are essential for accurate readings. Regular calibration ensures reliable results.
Experimental Setup
The experimental setup should minimize heat loss and ensure uniform heating to prevent localized temperature variations.
Beyond the Standard Boiling Point: Exploring Extreme Conditions
While 373.15 K is the standard boiling point, exploring water's behavior under extreme conditions reveals fascinating insights:
Superheated Water
Under certain conditions, water can exist as a superheated liquid, a metastable state where it remains liquid even above its normal boiling point. This occurs when heating is rapid and nucleation sites (points where vapor bubbles form) are absent.
Water at High Pressure
At extremely high pressures, the boiling point of water increases significantly. This is because the higher pressure necessitates a higher vapor pressure to initiate boiling.
Conclusion: The Significance of Water's Boiling Point
The boiling point of water in Kelvin, 373.15 K, is more than just a numerical value; it represents a fundamental property with far-reaching implications across diverse fields. Understanding this value, the underlying physics, and the factors that influence it is essential for appreciating the behavior of matter and its role in various scientific and practical applications. From cooking to industrial processes, and from scientific research to everyday life, the boiling point of water continues to hold a crucial position in our understanding of the world around us. Further exploration into the nuances of phase transitions and their dependence on temperature and pressure will undoubtedly lead to further advancements in science and technology.
Latest Posts
Latest Posts
-
Greatest Common Factor 27 And 36
Mar 10, 2025
-
What Is Prime Factorization Of 225
Mar 10, 2025
-
Tool Used To Detect Electric Charge
Mar 10, 2025
-
Formula For Perimeter Of A Polygon
Mar 10, 2025
-
Is Steel A Homogeneous Or Heterogeneous Mixture
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Boiling Point Of Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
