Is Steel A Homogeneous Or Heterogeneous Mixture
Juapaving
Mar 10, 2025 · 5 min read
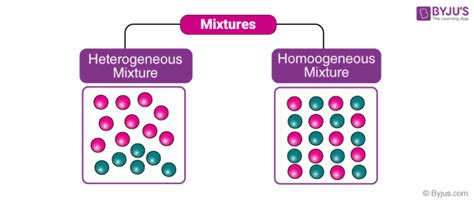
Table of Contents
Is Steel a Homogeneous or Heterogeneous Mixture? A Deep Dive into Material Science
Steel, a ubiquitous material shaping our modern world, often sparks the question: is it a homogeneous or heterogeneous mixture? The answer, while seemingly straightforward, delves into the fascinating intricacies of material science and the very definition of homogeneity and heterogeneity. This comprehensive exploration will unravel the complexities surrounding steel's microstructure, examining its components, properties, and the nuances that influence its classification.
Understanding Homogeneous and Heterogeneous Mixtures
Before diving into the specifics of steel, let's establish a clear understanding of the terms "homogeneous" and "heterogeneous." In chemistry and materials science, these terms describe the uniformity of a mixture's composition.
-
Homogeneous Mixture: A homogeneous mixture has a uniform composition throughout. This means that the constituent components are evenly distributed at a microscopic level, and the properties are consistent regardless of the sample location. Examples include saltwater, air (a mixture of gases), and many alloys under specific conditions.
-
Heterogeneous Mixture: A heterogeneous mixture exhibits a non-uniform composition. Different components are visibly distinguishable, and their properties vary depending on the sample location. Examples include sand and water, oil and water, and granite.
The Composition of Steel: A Complex Alloy
Steel is an alloy primarily composed of iron (Fe) and carbon (C). However, its composition doesn't stop there. Depending on the intended properties and application, steel can incorporate various other elements, including:
- Manganese (Mn): Improves strength and hardenability.
- Silicon (Si): Controls deoxidation during steelmaking and affects strength.
- Phosphorus (P): Often present as an impurity, but can affect properties at higher concentrations.
- Sulfur (S): Another impurity that negatively impacts steel's machinability.
- Chromium (Cr): Adds corrosion resistance (stainless steels).
- Nickel (Ni): Enhances strength, toughness, and corrosion resistance.
- Molybdenum (Mo): Increases hardenability and high-temperature strength.
- Vanadium (V): Refines grain size and enhances strength.
- Other alloying elements: Depending on the specific steel grade, numerous other elements might be present in smaller quantities.
The Microstructure: Key to Understanding Homogeneity
The key to determining whether steel is homogeneous or heterogeneous lies in its microstructure. This refers to the arrangement and distribution of the different phases present within the material at a microscopic level. Steel's microstructure is heavily influenced by factors such as:
- Carbon content: The amount of carbon significantly impacts the phases present and the resulting properties.
- Cooling rate: The speed at which molten steel cools affects the size and distribution of the phases. Rapid cooling often leads to finer microstructures, while slow cooling allows for larger structures to form.
- Alloying elements: The presence of additional elements can significantly alter the microstructure and the phases that precipitate out.
Phases in Steel
Depending on the carbon content and the cooling rate, different phases can exist in steel:
- Ferrite (α-iron): A body-centered cubic (BCC) structure, relatively soft and ductile. Dominant in low-carbon steels.
- Austenite (γ-iron): A face-centered cubic (FCC) structure, more ductile than ferrite. Present at higher temperatures and in higher-carbon steels.
- Pearlite: A lamellar structure composed of alternating layers of ferrite and cementite (Fe₃C). Forms during the slow cooling of medium-carbon steels.
- Martensite: A hard, brittle phase formed by rapid cooling of austenite. Characterizes high-carbon steels.
- Cementite: An iron carbide, hard and brittle.
The Case for Homogeneity (at a Macro Level)
In many practical applications, steel can be considered homogeneous at a macroscopic level. This means that its properties are relatively uniform across a large scale, making it suitable for engineering applications. When examined with the naked eye or even a low-power microscope, the various phases might not be readily distinguishable. The composition and properties appear consistent throughout the bulk material. The average composition is relatively uniform, leading to consistent mechanical behavior.
The Case for Heterogeneity (at a Micro Level)
At a microscopic level, however, the picture changes. Steel’s microstructure isn't truly uniform. Different phases, each with its unique properties, coexist. The distribution of these phases isn't perfectly even, resulting in slight variations in the material's properties at a microscopic scale. The size, shape, and distribution of these phases can vary significantly, depending on the processing history of the steel. This inherent variation is a key factor influencing the material's mechanical characteristics and justifies considering steel as heterogeneous at a microscopic scale. Examining a polished and etched sample under a microscope reveals the heterogeneous nature – distinct regions of differing phases are clearly visible.
The Role of Heat Treatment
Heat treatment processes are crucial in manipulating steel's microstructure and thus, its macroscopic properties. These processes involve heating and cooling the steel under controlled conditions to alter the distribution and size of the phases. For instance:
- Annealing: Reduces internal stresses and improves ductility.
- Quenching: Rapid cooling to transform austenite into martensite, increasing hardness.
- Tempering: Heating martensite to reduce brittleness while maintaining high hardness.
These processes highlight the dynamic nature of steel's microstructure and the ability to manipulate its homogeneity (at a macroscopic level) through controlled thermomechanical treatments.
Conclusion: A Matter of Scale and Perspective
The question of whether steel is homogeneous or heterogeneous is not simply a "yes" or "no" answer. It depends entirely on the scale at which the material is examined. At a macroscopic level, for most practical engineering applications, steel behaves as a homogeneous material with consistent properties. Its average composition and overall mechanical behavior are reasonably uniform across larger volumes. However, at a microscopic level, the presence of various phases, their non-uniform distribution, and the variation in their arrangement definitively make steel a heterogeneous mixture. Understanding this dual nature is crucial for effectively utilizing steel's diverse properties in various engineering and industrial applications. The heterogeneous nature at the micro level influences the overall macroscopic properties through cumulative effects of the individual phases and their interactions. Therefore, acknowledging both aspects – macroscopic homogeneity and microscopic heterogeneity – provides a complete understanding of this versatile material.
Latest Posts
Latest Posts
-
Why Is The Earth Referred To As The Blue Planet
Mar 10, 2025
-
What Fraction Is Equal To 3 6
Mar 10, 2025
-
Two Or More Elements Chemically Combined
Mar 10, 2025
-
Which Of The Following Is A Primary Activity
Mar 10, 2025
-
What Is The Prime Factorization Of 34
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Is Steel A Homogeneous Or Heterogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
