Blood Acquires Its Red Colour From
Juapaving
Mar 21, 2025 · 6 min read
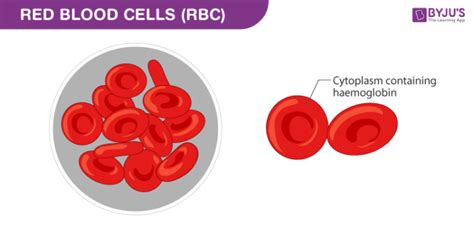
Table of Contents
Blood Acquires Its Red Color From: Hemoglobin and the Magic of Iron
Blood, the vibrant crimson fluid coursing through our veins and arteries, is far more than just a simple liquid. It's a complex, dynamic transportation system, crucial for life itself. And its striking red color? That's thanks to a remarkable protein called hemoglobin, and the essential role of iron within its structure. This article delves deep into the fascinating science behind blood's crimson hue, exploring the chemistry, biology, and even the implications of variations in blood color.
The Star of the Show: Hemoglobin
Hemoglobin is a metalloprotein found within red blood cells, or erythrocytes. These tiny, biconcave discs are responsible for carrying oxygen throughout the body. Hemoglobin's primary function is oxygen transport, but it's the way it achieves this that explains blood's distinctive color.
Hemoglobin Structure: A Complex Assembly
Hemoglobin's structure is remarkably intricate. Each hemoglobin molecule is composed of four subunits: two alpha (α) and two beta (β) globin chains. Each of these globin chains cradles a heme group, the key player in oxygen binding and the source of blood's red color.
The Heme Group: Iron's Crucial Role
The heme group is a porphyrin ring complex containing a single iron (Fe) ion at its center. This iron ion is the vital component that allows hemoglobin to bind to and transport oxygen. The iron atom in its ferrous (Fe²⁺) state can reversibly bind to an oxygen molecule (O₂). This binding process is what gives blood its characteristic red color.
Oxygenation and Color Change: A Dynamic Equilibrium
When hemoglobin binds to oxygen in the lungs, it forms oxyhemoglobin. This oxygenated form of hemoglobin is bright red, giving oxygenated blood its characteristic scarlet hue. This bright red color is due to the change in the electronic configuration of the iron ion upon oxygen binding, influencing the way the molecule interacts with light.
Conversely, when hemoglobin releases oxygen in the body's tissues, it becomes deoxyhemoglobin. Deoxyhemoglobin has a much darker, purplish-red color. This difference in color is readily observable in veins, which carry deoxygenated blood back to the lungs.
The Chemistry of Color: Light Absorption and Reflection
The color of blood is not simply a matter of pigment; it's a result of the way hemoglobin interacts with light. Hemoglobin molecules absorb certain wavelengths of light and reflect others. Oxyhemoglobin absorbs more green and blue light, reflecting primarily red light, thus appearing bright red. Deoxyhemoglobin, on the other hand, absorbs less red light and more other wavelengths, resulting in its darker, purplish-red appearance.
This interaction with light is precisely measurable using spectrophotometry, a technique that measures the amount of light absorbed or transmitted by a solution at different wavelengths. This helps scientists understand the differences in the absorption spectra of oxyhemoglobin and deoxyhemoglobin.
Variations in Blood Color: Beyond Red
While the characteristic red color of blood is predominantly due to hemoglobin and its iron content, there can be variations in the shade and intensity of this color. Several factors can influence this:
-
Oxygen Saturation: The percentage of hemoglobin molecules bound to oxygen directly affects the color. High oxygen saturation results in a brighter red, while low saturation leads to a darker red or purplish hue.
-
Hemoglobin Concentration: Higher hemoglobin concentrations lead to a deeper, more intense red color. Anemia, characterized by low hemoglobin levels, results in paler blood.
-
Presence of other pigments: Other substances in the blood, though present in much smaller quantities than hemoglobin, can influence the overall color. For example, bilirubin, a byproduct of red blood cell breakdown, can give blood a yellowish tinge in certain conditions such as jaundice.
-
Blood vessel characteristics: The appearance of blood can also be affected by the type of blood vessel being observed. Arteries, with their oxygen-rich blood, tend to show a brighter red, while veins, carrying deoxygenated blood, may appear darker.
The Importance of Iron: Beyond Hemoglobin
Iron's role in blood's red color is undeniable, but its importance extends far beyond simply determining the shade of blood. Iron is an essential micronutrient, vital for numerous bodily functions. Its deficiency can lead to anemia, a condition characterized by reduced red blood cell production and decreased oxygen-carrying capacity. The symptoms of iron-deficiency anemia often include fatigue, weakness, and shortness of breath.
Iron's crucial role in various enzymes and proteins: Iron is not only found in hemoglobin; it's also a component of other essential molecules, including myoglobin (oxygen-carrying protein in muscle tissue), cytochromes (involved in cellular respiration), and numerous enzymes. These enzymes participate in a variety of metabolic processes, highlighting iron's importance in overall health and well-being.
Beyond the Basics: Advanced Concepts
The study of hemoglobin and blood color involves far more complex interactions than what has been briefly described here. Here are a few advanced concepts to consider:
-
Allosteric Regulation: The binding of oxygen to one heme group in hemoglobin influences the binding affinity of oxygen to other heme groups in the same molecule. This cooperativity is essential for efficient oxygen uptake and release.
-
Bohr Effect: The pH of the blood affects hemoglobin's affinity for oxygen. A decrease in pH (more acidic) reduces the affinity, promoting oxygen release in tissues with high metabolic activity.
-
2,3-Bisphosphoglycerate (2,3-BPG): This molecule binds to deoxyhemoglobin, reducing its affinity for oxygen and facilitating oxygen release in tissues.
-
Carbon Monoxide Poisoning: Carbon monoxide (CO) binds to hemoglobin with much higher affinity than oxygen, preventing oxygen transport and leading to potentially fatal consequences. The resulting carboxyhemoglobin has a bright cherry-red color, which can be misleading.
-
Genetic Variations in Hemoglobin: Mutations in the genes encoding globin chains can lead to various hemoglobinopathies, such as sickle cell anemia and thalassemia, affecting both blood color and function.
The Ongoing Research: Unraveling the Mysteries
Research on hemoglobin and blood continues to advance our understanding of its structure, function, and related disorders. Scientists are actively investigating new ways to diagnose and treat blood-related conditions, improve oxygen therapy, and develop novel diagnostic tools. The understanding of hemoglobin's intricate mechanism allows us to understand how to improve the efficiency of blood transfusions and explore potential therapies for various blood-related diseases.
Conclusion: A Symphony of Iron and Protein
The red color of blood is a testament to the remarkable properties of hemoglobin and the vital role of iron. Understanding the chemistry and biology behind this vibrant hue offers a window into the complex mechanisms that sustain life. Further research promises to uncover even more intricacies within this vital bodily fluid, improving our understanding of health and disease. The red color of blood is more than just a visual characteristic; it is a reflection of the intricate and fascinating biology of life itself.
Latest Posts
Latest Posts
-
Sum Of Interior Angles Of A Dodecagon
Mar 22, 2025
-
What Is The Factors Of 91
Mar 22, 2025
-
What Is The Molar Mass Of P2o5
Mar 22, 2025
-
What Is The Lcm Of 3 9 12
Mar 22, 2025
-
A Magnetic Field Induced In The Conductor Carrying The Current
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Blood Acquires Its Red Colour From . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
