What Is The Molar Mass Of P2o5
Juapaving
Mar 22, 2025 · 5 min read
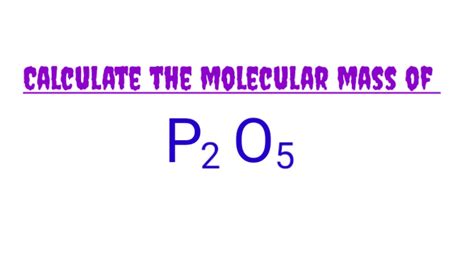
Table of Contents
What is the Molar Mass of P₂O₅? A Deep Dive into Phosphorus Pentoxide
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This article delves into the specifics of calculating the molar mass of phosphorus pentoxide (P₂O₅), explaining the process step-by-step and exploring its relevance in different chemical contexts. We'll also touch upon the properties and applications of this important compound.
Understanding Molar Mass
Before we calculate the molar mass of P₂O₅, let's clarify the concept. Molar mass is the mass of one mole of a substance. A mole is a unit of measurement in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). The molar mass is essentially the sum of the atomic masses of all atoms in a molecule, expressed in grams per mole (g/mol).
Atomic Masses: The Building Blocks
The foundation of molar mass calculation lies in the atomic masses of the elements involved. These values are typically found on the periodic table. For our calculation of P₂O₅, we need the atomic masses of phosphorus (P) and oxygen (O).
- Phosphorus (P): The atomic mass of phosphorus is approximately 30.97 g/mol.
- Oxygen (O): The atomic mass of oxygen is approximately 16.00 g/mol.
Calculating the Molar Mass of P₂O₅
Now, let's calculate the molar mass of P₂O₅:
-
Identify the number of atoms of each element: The chemical formula P₂O₅ indicates that there are two phosphorus atoms and five oxygen atoms in one molecule of phosphorus pentoxide.
-
Multiply the atomic mass of each element by its number of atoms:
- Phosphorus: 2 atoms × 30.97 g/mol/atom = 61.94 g/mol
- Oxygen: 5 atoms × 16.00 g/mol/atom = 80.00 g/mol
-
Add the results together:
- Total molar mass of P₂O₅ = 61.94 g/mol + 80.00 g/mol = 141.94 g/mol
Therefore, the molar mass of P₂O₅ is approximately 141.94 grams per mole. This means that one mole of P₂O₅ weighs approximately 141.94 grams.
Significance of Molar Mass in Chemistry
The molar mass of P₂O₅, like the molar mass of any compound, holds significant importance in various chemical calculations and applications:
1. Stoichiometric Calculations:
Molar mass is essential for stoichiometric calculations, which involve determining the quantitative relationships between reactants and products in chemical reactions. Knowing the molar mass allows us to convert between mass and moles, enabling us to predict the amount of product formed or reactant consumed in a reaction.
2. Solution Chemistry:
In solution chemistry, molar mass is crucial for preparing solutions of a specific concentration. For example, to prepare a solution of a known molarity (moles per liter), we need to know the molar mass to accurately weigh out the required amount of solute.
3. Gas Law Calculations:
The ideal gas law (PV = nRT) relates the pressure, volume, temperature, and number of moles of a gas. Molar mass is used to convert the mass of a gas to moles, enabling calculations of other gas parameters.
4. Determining Empirical and Molecular Formulas:
Molar mass is an important piece of information when determining the empirical and molecular formulas of compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms in a molecule.
Properties and Applications of P₂O₅
Phosphorus pentoxide, a powerful dehydrating agent, possesses several distinctive properties and finds wide applications in various fields:
1. Dehydrating Agent:
Its strong affinity for water makes P₂O₅ an exceptionally effective dehydrating agent. It's often used to dry gases and solvents, removing traces of moisture. This property is exploited in various chemical processes requiring anhydrous conditions.
2. Synthesis of Phosphoric Acid:
P₂O₅ is a crucial intermediate in the industrial production of phosphoric acid (H₃PO₄), a vital compound in fertilizers and various other applications. The reaction involves the controlled hydration of P₂O₅.
3. Synthesis of Organic Compounds:
P₂O₅'s dehydrating properties also find use in the synthesis of certain organic compounds. It can facilitate dehydration reactions, leading to the formation of various organic molecules.
4. Niches Applications:
Beyond its major applications, P₂O₅ finds niche uses in areas such as:
- Food Industry: (though less common due to toxicity concerns) It has been used as a food additive (E-number E339) in some processed foods.
- Polymer Chemistry: It can be involved in certain polymerization reactions.
Safety Precautions: Handling P₂O₅
Phosphorus pentoxide is a highly reactive compound and requires careful handling. It reacts violently with water, producing significant heat. Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat when handling P₂O₅. Work in a well-ventilated area or under a fume hood to avoid inhaling the dust.
Conclusion
The molar mass of P₂O₅, calculated as 141.94 g/mol, serves as a fundamental parameter in various chemical calculations. Understanding this value is essential for accurate stoichiometric analyses, solution preparation, gas law calculations, and other critical chemical processes. Its properties as a powerful dehydrating agent contribute to its widespread applications across numerous fields, but its reactivity necessitates careful handling and appropriate safety measures. The importance of precise molar mass calculations extends beyond this specific compound, emphasizing the foundational role of this concept in chemistry. By understanding and applying the principles of molar mass calculation, chemists can accurately predict reaction outcomes, synthesize compounds effectively, and ensure safe laboratory practices.
Latest Posts
Latest Posts
-
What Describes The Outcome Of Mitosis
Mar 23, 2025
-
Five Letter Words With E R At The End
Mar 23, 2025
-
The Numerical Factor In A Term
Mar 23, 2025
-
A Subset Or Part Of A Population
Mar 23, 2025
-
What Is The Lcm Of 3 6 And 9
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of P2o5 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
