Based On The Law Of Conservation Of Energy
Juapaving
Mar 22, 2025 · 6 min read
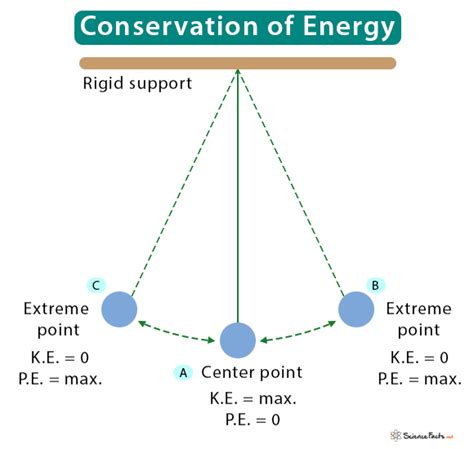
Table of Contents
- Based On The Law Of Conservation Of Energy
- Table of Contents
- Based on the Law of Conservation of Energy: A Deep Dive into Physics' Fundamental Principle
- Understanding the Law: More Than Just a Simple Statement
- Forms of Energy: A Diverse Family
- The Law in Action: Everyday Examples
- The Importance of Efficiency and Losses
- Beyond the Basics: Advanced Concepts and Implications
- Internal Energy and Thermodynamics
- Mass-Energy Equivalence (E=mc²)
- Open and Closed Systems
- Limitations and Exceptions?
- Applications: A Wide-Ranging Impact
- Conclusion: A Timeless Principle
- Latest Posts
- Latest Posts
- Related Post
Based on the Law of Conservation of Energy: A Deep Dive into Physics' Fundamental Principle
The Law of Conservation of Energy, a cornerstone of physics, states that energy cannot be created or destroyed, only transformed from one form to another. This seemingly simple statement underpins our understanding of the universe, from the smallest subatomic particles to the largest celestial bodies. This article will delve deep into this fundamental law, exploring its implications, applications, and the subtle nuances that often accompany its interpretation.
Understanding the Law: More Than Just a Simple Statement
The law's simplicity is deceptive. It's not merely stating that the total energy remains constant; it specifies that energy transforms. This transformation is crucial. Energy can manifest in numerous forms, each with its unique characteristics and interactions:
Forms of Energy: A Diverse Family
-
Kinetic Energy: The energy of motion. A moving car, a flowing river, even the vibrating atoms within a solid all possess kinetic energy. Its magnitude depends on mass and velocity (KE = 1/2mv²).
-
Potential Energy: Stored energy due to position or configuration. A ball held high above the ground possesses gravitational potential energy, ready to be converted into kinetic energy as it falls. A stretched spring holds elastic potential energy. Chemical bonds store chemical potential energy.
-
Thermal Energy (Heat): The internal energy of a system related to the random motion of its particles. Heat transfer involves the flow of thermal energy from a hotter object to a colder one.
-
Radiant Energy (Electromagnetic Radiation): Energy carried by electromagnetic waves, including light, radio waves, X-rays, and gamma rays. The sun's energy reaches Earth as radiant energy.
-
Nuclear Energy: Energy stored within the nucleus of an atom. Nuclear fission and fusion release enormous amounts of nuclear energy.
-
Electrical Energy: Energy associated with the flow of electric charge. This powers our homes and industries.
-
Sound Energy: Energy transmitted through vibrations in a medium, such as air or water.
-
Chemical Energy: Energy stored in the bonds between atoms in molecules. This is released during chemical reactions, such as combustion.
The Law in Action: Everyday Examples
The law of conservation of energy is constantly at play in our everyday lives. Consider these examples:
-
A Rollercoaster: At the top of the hill, the rollercoaster possesses maximum potential energy. As it descends, this potential energy converts into kinetic energy, increasing its speed. Friction and air resistance cause some energy to dissipate as heat, but the total energy remains relatively constant throughout the ride.
-
Burning a Candle: The chemical potential energy stored within the wax is released as heat and light during combustion. This energy is not destroyed; it's transformed into different forms.
-
Photosynthesis: Plants convert radiant energy from sunlight into chemical potential energy stored in glucose molecules. This process is vital for life on Earth.
-
Hydroelectric Power Plants: The potential energy of water stored behind a dam is converted into kinetic energy as the water flows down, turning turbines and generating electricity.
The Importance of Efficiency and Losses
While the total energy remains constant, the useful energy often decreases during energy transformations. This is due to energy losses, primarily in the form of heat:
-
Friction: Whenever two surfaces rub against each other, some kinetic energy is converted into thermal energy, leading to a loss of useful energy.
-
Air Resistance (Drag): Moving objects experience resistance from the air, converting kinetic energy into thermal energy.
-
Inefficient Machines: Real-world machines are never perfectly efficient. Some energy is always lost as heat due to friction and other internal losses.
These losses highlight the importance of energy efficiency. Designing more efficient machines and processes reduces wasted energy, leading to environmental and economic benefits.
Beyond the Basics: Advanced Concepts and Implications
The simple statement of the law needs careful consideration in more advanced contexts:
Internal Energy and Thermodynamics
In thermodynamics, the focus shifts to the internal energy of a system – the sum of its kinetic and potential energies at a microscopic level. The first law of thermodynamics is essentially a restatement of the law of conservation of energy, considering internal energy changes and heat and work transfers.
Mass-Energy Equivalence (E=mc²)
Einstein's famous equation, E=mc², demonstrates a profound connection between energy and mass. This equation reveals that mass itself is a form of energy, and a small amount of mass can be converted into a tremendous amount of energy, as seen in nuclear reactions. This extends the law of conservation of energy to encompass mass-energy.
Open and Closed Systems
The law applies differently to open and closed systems. A closed system does not exchange energy or matter with its surroundings, maintaining a constant total energy. An open system, however, can exchange both, making the total energy within the system variable.
Limitations and Exceptions?
The law of conservation of energy is incredibly robust, holding true across a vast range of phenomena. However, it's important to note that it applies primarily to classical mechanics. In some advanced areas of physics, such as quantum mechanics and general relativity, the law might require more nuanced interpretations or even slight modifications under extremely specific circumstances, mainly involving the creation and annihilation of particles.
Applications: A Wide-Ranging Impact
The implications of the law of conservation of energy are far-reaching and touch upon numerous aspects of our lives and technological advancements:
-
Energy Production: Understanding how energy transforms is fundamental to developing efficient energy production methods, from fossil fuels to renewable sources like solar, wind, and hydroelectric power.
-
Engineering Design: Engineers apply the law to design efficient machines, vehicles, and structures, minimizing energy waste and maximizing performance.
-
Climate Change Mitigation: Understanding energy transformations and losses is crucial for developing strategies to mitigate climate change by reducing greenhouse gas emissions and promoting sustainable energy use.
-
Medical Imaging: Techniques like MRI and PET scans rely on principles of energy transformations within the body.
-
Space Exploration: Rocket propulsion, satellite operation, and space exploration all heavily depend on converting various forms of energy to achieve their objectives.
Conclusion: A Timeless Principle
The law of conservation of energy stands as a testament to the elegance and underlying unity of the physical world. Its implications are vast, shaping our understanding of the universe and driving technological advancements. While its simple statement belies the complexity and breadth of its applications, the principle remains a cornerstone of physics, guiding research and development across a wide range of disciplines and informing our daily lives in countless ways. Further study into its nuances and implications promises even greater insights into the workings of nature and the possibilities it offers for human innovation. Continuous exploration of this fundamental law ensures our ability to harness energy efficiently and sustainably for a better future.
Latest Posts
Latest Posts
-
Is Air A Compound Mixture Or Element
Mar 24, 2025
-
Displacement Is Which Of The Following Types Of Quantities
Mar 24, 2025
-
What Is The Lcm Of 5 And 20
Mar 24, 2025
-
What Is The Advantage Of Ac Over Dc
Mar 24, 2025
-
A Rhombus With 4 Right Angles
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Based On The Law Of Conservation Of Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
