At What Temperature Does Water Boil Celsius
Juapaving
Mar 06, 2025 · 5 min read
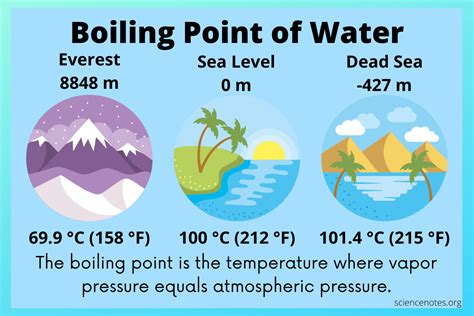
Table of Contents
At What Temperature Does Water Boil Celsius? A Deep Dive into Boiling Point
The seemingly simple question, "At what temperature does water boil Celsius?" opens a door to a fascinating exploration of physics, chemistry, and the properties of water. While the short answer is 100°C at standard atmospheric pressure, the reality is far more nuanced and depends on several factors. This comprehensive article delves into the intricacies of water's boiling point, examining the science behind it and exploring the variables that can influence this crucial temperature.
Understanding Boiling Point: A Fundamental Concept
The boiling point of a liquid is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. In simpler terms, it's the temperature at which bubbles of vapor begin to form within the liquid and rise to the surface. This is different from evaporation, which is the slower process of molecules escaping from the liquid's surface. Boiling is a much more energetic and rapid phase transition.
For water, under standard atmospheric pressure (defined as 1 atmosphere or 101.325 kPa), this transition occurs at exactly 100°C (212°F). This is a fundamental constant often used in scientific calculations and everyday life. However, this seemingly simple fact is heavily dependent on the environment in which the water is heated.
The Role of Atmospheric Pressure
Atmospheric pressure is the weight of the air above a given point. At higher altitudes, where there's less air above, the atmospheric pressure is lower. This has a direct impact on water's boiling point. Because lower pressure requires less energy for the vapor pressure to equal it, water boils at a lower temperature at higher altitudes. Conversely, at higher pressures (like in a pressure cooker), water boils at a higher temperature.
This relationship is crucial in understanding why cooking times differ at various altitudes. At higher altitudes, water boils quicker but at a lower temperature, meaning food takes longer to cook. Conversely, at lower altitudes or in pressure cookers, the higher boiling point allows for faster cooking.
Factors influencing atmospheric pressure and therefore the boiling point:
- Altitude: The most significant factor; higher altitude means lower pressure and lower boiling point.
- Weather conditions: High pressure systems can slightly elevate the boiling point, while low-pressure systems can lower it.
- Geographic location: Subtle variations in atmospheric pressure exist across different geographical regions.
Impurities and Boiling Point: The Unexpected Effect
While pure water boils at 100°C under standard pressure, the presence of impurities can subtly alter this temperature. These impurities, which could be dissolved salts, minerals, or other substances, can affect the boiling point through a phenomenon called boiling point elevation.
Boiling point elevation is a colligative property, meaning it depends on the concentration of solute particles rather than their identity. Essentially, the dissolved impurities interfere with the water molecules' ability to escape into the vapor phase, requiring a slightly higher temperature for boiling to occur.
This effect is usually small for most everyday scenarios, but it's significant in certain industrial processes and scientific experiments where precise boiling points are crucial. For instance, saltwater will boil at a slightly higher temperature than pure water.
Types of impurities and their effect:
- Dissolved salts: These significantly elevate the boiling point, even in small concentrations.
- Minerals: Similar to salts, minerals in hard water can influence the boiling point.
- Other dissolved substances: Any dissolved substance will contribute to boiling point elevation, albeit to varying degrees.
Other Factors Affecting Water's Boiling Point
Beyond atmospheric pressure and impurities, several other factors, albeit minor, can influence the temperature at which water boils:
- Container material: While the effect is negligible, the material of the container can subtly affect heat transfer and, therefore, the observed boiling point.
- Heat source: The efficiency and uniformity of the heat source can influence how quickly water reaches its boiling point, but not the boiling point itself.
- Volume of water: A larger volume of water might take longer to boil, but the boiling point remains unchanged.
- Presence of nucleation sites: These are tiny imperfections or irregularities on the container's surface where bubbles can more easily form. A smooth container might lead to superheating, where the water temporarily exceeds its boiling point before suddenly boiling violently.
The Importance of Accurate Boiling Point Measurement
Precise measurements of boiling point are critical in several fields:
- Chemistry and Physics: Boiling point is a crucial physical property used to identify and characterize substances.
- Food science and culinary arts: Understanding how altitude and impurities affect boiling point is essential for consistent cooking results.
- Industrial processes: Many industrial processes rely on precise temperature control, including distillation and sterilization.
- Meteorology: Boiling point measurements can be used to infer atmospheric pressure and altitude.
Beyond the Basics: Exploring Superheating and Subcooling
While 100°C under standard conditions is the typical boiling point, water can sometimes exhibit unusual behavior:
- Superheating: This occurs when water is heated above its boiling point without boiling. It usually requires a very smooth, clean container and careful heating to prevent bubble nucleation. Once boiling begins, it can be quite violent.
- Subcooling: This is the opposite phenomenon where water is cooled below its freezing point (0°C) without freezing. Like superheating, it requires very pure water and careful cooling to prevent ice crystal formation.
Conclusion: The Dynamic Nature of Water's Boiling Point
The simple question, "At what temperature does water boil Celsius?" leads to a complex and fascinating answer. While the standard answer is 100°C at standard atmospheric pressure, the actual boiling point is influenced by a variety of factors, including atmospheric pressure, impurities, and the presence of nucleation sites. Understanding these variables is crucial in various scientific, culinary, and industrial applications. By appreciating the dynamic nature of water's boiling point, we gain a deeper understanding of this fundamental physical property and its far-reaching implications. The seemingly simple act of boiling water reveals a world of intricate scientific principles, underscoring the beauty and complexity of the natural world. Further research into the specific conditions and variables can lead to a more precise understanding of this critical temperature in specific scenarios. Therefore, while 100°C serves as a reliable benchmark, it's essential to remember the nuances that affect the precise boiling point of water in real-world situations.
Latest Posts
Latest Posts
-
Common Multiples Of 2 And 7
Mar 06, 2025
-
What Does The Arrow In A Food Chain Represent
Mar 06, 2025
-
What Is The Least Common Multiple Of 9 And 15
Mar 06, 2025
-
Rate Constant Varies With Temperature By Equation
Mar 06, 2025
-
Least Common Multiple For 9 And 15
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about At What Temperature Does Water Boil Celsius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
