Rate Constant Varies With Temperature By Equation
Juapaving
Mar 06, 2025 · 6 min read
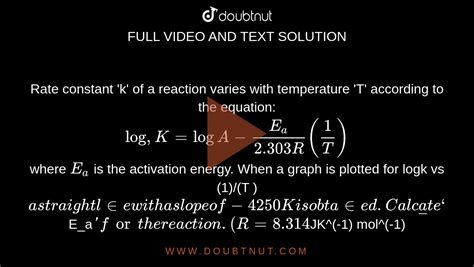
Table of Contents
How the Rate Constant Varies with Temperature: An In-Depth Look at the Arrhenius Equation and Beyond
The rate of a chemical reaction is rarely constant; it's highly sensitive to changes in conditions, with temperature being a particularly significant factor. Understanding how temperature affects reaction rates is crucial in various fields, from industrial chemistry and materials science to environmental science and biochemistry. This relationship is primarily governed by the Arrhenius equation, a cornerstone of chemical kinetics. This article delves deep into the Arrhenius equation, exploring its implications, limitations, and extensions. We'll also examine how collision theory and transition state theory provide a more nuanced understanding of this temperature dependence.
The Arrhenius Equation: A Quantitative Relationship
The Arrhenius equation provides a quantitative description of the relationship between the rate constant (k) of a reaction and temperature (T):
k = A * exp(-Ea/RT)
Where:
- k is the rate constant (usually in units of s⁻¹, or with units reflecting the overall reaction order)
- A is the pre-exponential factor (or frequency factor), representing the frequency of collisions with the correct orientation for a reaction to occur. It has the same units as the rate constant.
- Ea is the activation energy, the minimum energy required for the reactants to overcome the energy barrier and form products. It's expressed in Joules per mole (J/mol) or kilocalories per mole (kcal/mol).
- R is the ideal gas constant (8.314 J/mol·K)
- T is the absolute temperature in Kelvin (K)
The exponential term, exp(-Ea/RT), represents the fraction of molecules possessing sufficient energy to react at a given temperature. As temperature increases, this fraction increases exponentially, leading to a significant increase in the rate constant.
Understanding the Components of the Arrhenius Equation
Let's examine each component in more detail:
-
Activation Energy (Ea): This is arguably the most important parameter in the Arrhenius equation. A higher activation energy implies a slower reaction at a given temperature, as a larger fraction of molecules needs to possess sufficient energy to react. Ea provides insight into the reaction mechanism and the nature of the transition state.
-
Pre-exponential Factor (A): This factor accounts for the frequency of collisions with the correct orientation for reaction. It's influenced by factors like steric effects (molecular geometry and orientation) and collision frequency. A larger A value indicates a greater probability of successful collisions, resulting in a faster reaction rate.
-
Temperature (T): The absolute temperature plays a crucial role in determining the reaction rate. A higher temperature increases the kinetic energy of the molecules, leading to more frequent and energetic collisions, and thus a higher rate constant.
Experimental Determination of the Arrhenius Parameters
The Arrhenius parameters (A and Ea) can be determined experimentally by measuring the rate constant at different temperatures. The most common method involves plotting ln(k) against 1/T, which yields a straight line according to the linearized form of the Arrhenius equation:
ln(k) = ln(A) - Ea/RT
This linear form is obtained by taking the natural logarithm of the Arrhenius equation. The slope of the line is -Ea/R, allowing for the calculation of the activation energy. The y-intercept is ln(A), from which the pre-exponential factor can be determined.
Limitations of the Arrhenius Equation
While the Arrhenius equation is remarkably successful in describing the temperature dependence of many reactions, it has some limitations:
- Simplicity: It assumes a constant activation energy over the temperature range considered. In reality, activation energy can vary slightly with temperature.
- Specific Reactions: It's primarily applicable to elementary reactions (single-step reactions). For complex reactions involving multiple steps, the overall temperature dependence might be more complicated.
- Idealized Conditions: It assumes ideal behavior, neglecting factors like solvent effects, ionic strength, and non-ideal gas behavior.
Beyond the Arrhenius Equation: Collision Theory and Transition State Theory
The Arrhenius equation provides an empirical relationship, but collision theory and transition state theory offer deeper mechanistic insights into the temperature dependence of reaction rates.
Collision Theory
Collision theory postulates that for a reaction to occur, reactant molecules must collide with sufficient energy and proper orientation. The rate constant is related to the collision frequency (Z), the fraction of collisions with sufficient energy (f), and the steric factor (p) which accounts for the correct orientation:
k = Z * p * f
Temperature affects both the collision frequency (increasing with temperature) and the fraction of molecules with sufficient energy (also increasing exponentially with temperature). This provides a microscopic explanation for the Arrhenius behavior.
Transition State Theory (TST)
Transition state theory provides a more sophisticated model by considering the formation of an activated complex or transition state – a high-energy intermediate species formed during the reaction. TST introduces the concept of the Gibbs free energy of activation (ΔG‡), enthalpy of activation (ΔH‡), and entropy of activation (ΔS‡). The rate constant is expressed as:
k = (k<sub>B</sub>T/h) * exp(-ΔG‡/RT)
where:
- k<sub>B</sub> is the Boltzmann constant
- h is Planck's constant
TST provides a more detailed picture, linking the rate constant to thermodynamic properties of the transition state. It accounts for the entropy of activation, reflecting the influence of molecular structure and orientation on the reaction rate. It’s particularly useful for understanding reactions in solution and complex environments.
Temperature Dependence in Different Reaction Orders
The temperature dependence described by the Arrhenius equation influences how the reaction rate changes over time for various reaction orders:
-
First-order reactions: The rate is directly proportional to the concentration of one reactant. Increasing temperature exponentially increases the rate constant, leading to a faster decrease in reactant concentration over time.
-
Second-order reactions: The rate depends on the concentration of two reactants (or the square of one reactant). While the temperature dependence is still governed by the Arrhenius equation, the overall effect on the reaction rate is more complex due to the concentration dependence.
-
Zero-order reactions: The rate is independent of the concentration of reactants (up to a certain concentration limit). In this unusual case, increasing temperature only affects the rate constant itself, thus directly influencing the reaction rate. Zero-order kinetics often indicate that the reaction rate is limited by factors other than reactant concentration.
Applications and Significance
The understanding of how rate constants vary with temperature has far-reaching applications:
- Industrial Chemistry: Optimizing reaction conditions to maximize yield and minimize reaction time.
- Catalysis: Designing catalysts that lower the activation energy, thereby increasing reaction rates at lower temperatures.
- Food Science: Predicting food spoilage rates and determining appropriate storage conditions.
- Environmental Science: Modeling the rates of atmospheric reactions and pollutant degradation.
- Pharmacokinetics: Determining the rate of drug metabolism and distribution in the body.
Conclusion
The Arrhenius equation provides a fundamental framework for understanding the temperature dependence of reaction rates. While it has limitations, it remains an invaluable tool in chemical kinetics. Collision theory and transition state theory offer more mechanistic insights, providing a deeper understanding of the factors influencing reaction rates at different temperatures. The applications of this knowledge are extensive, impacting numerous scientific and technological fields. Further research continues to refine our understanding of the complex interplay between temperature, reaction mechanisms, and rate constants, leading to new advancements in various scientific disciplines.
Latest Posts
Latest Posts
-
Is 23 A Composite Or Prime Number
Mar 06, 2025
-
Consecutive Angles In A Parallelogram Are
Mar 06, 2025
-
Is Root 72 A Rational Number
Mar 06, 2025
-
What Is The Prime Factorization Of 15
Mar 06, 2025
-
A Heat Flux Of 4000 J S Is To Be Passed
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about Rate Constant Varies With Temperature By Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
