As You Move Down A Group Atomic Radius Increases Because
Juapaving
Mar 19, 2025 · 6 min read
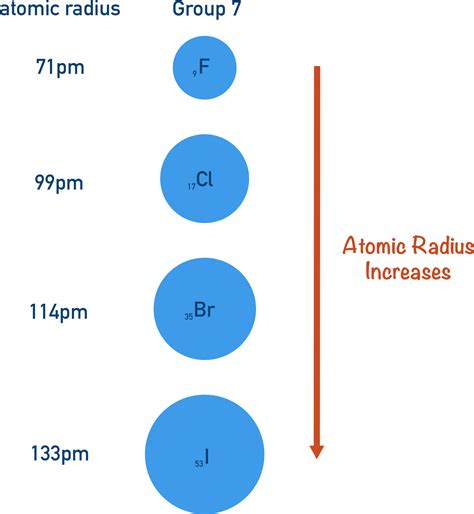
Table of Contents
As You Move Down a Group, Atomic Radius Increases: A Deep Dive into Periodic Trends
The periodic table, a seemingly simple arrangement of elements, holds within it a wealth of information about the behavior and properties of matter. One of the most fundamental trends observable is the increase in atomic radius as you move down a group (column) in the table. Understanding why this occurs requires a delve into the structure of the atom and the forces that govern its size. This article will explore this trend in detail, examining the underlying principles and addressing common misconceptions.
Understanding Atomic Radius
Before we dive into the reasons behind the increase in atomic radius down a group, let's first define what we mean by "atomic radius." It's not a straightforward measurement like the radius of a perfectly spherical ball. Instead, atomic radius is a measure of the size of an atom, typically defined as half the distance between the nuclei of two identical atoms that are just touching each other. This definition highlights the challenges in precisely measuring such a small entity, and the fact that the atomic radius isn't a fixed value but rather a range depending on the method of measurement and the specific bonding environment.
There are various ways to measure atomic radius, including covalent radius (half the distance between two covalently bonded atoms), metallic radius (half the distance between two adjacent atoms in a metallic crystal lattice), and van der Waals radius (half the distance between the nuclei of two non-bonded atoms). While there are differences between these radii, they all exhibit the same general trend: an increase down a group. For simplicity, we will focus on the general trend of atomic radius increase.
The Role of Electron Shells and Shielding
The primary reason for the increase in atomic radius as you move down a group is the addition of electron shells. As you progress down a group, each successive element adds an electron to a new, higher energy level shell, further from the nucleus. This is fundamental to the organization of the periodic table.
Think of it like this: imagine adding layers to an onion. Each layer represents a new electron shell. As you add layers, the overall size of the onion increases. Similarly, as you move down a group, adding a new electron shell pushes the outermost electrons further away from the nucleus, leading to a larger atomic radius.
The effect of electron shielding also plays a crucial role. Inner electrons partially shield the outermost (valence) electrons from the attractive force of the positively charged nucleus. The more inner electrons there are, the greater the shielding effect, reducing the net positive charge experienced by the valence electrons. This reduced effective nuclear charge allows the outermost electrons to be less tightly held, expanding the atomic radius. The increase in shielding outweighs the increase in nuclear charge down a group.
Shielding and Effective Nuclear Charge: A Closer Look
The concept of effective nuclear charge is crucial. While the number of protons in the nucleus (and therefore the positive charge) increases down a group, the increase in shielding effect by the inner electrons offsets this. The outermost electrons experience a smaller net positive charge, known as the effective nuclear charge (Z<sub>eff</sub>), than the actual nuclear charge (Z). This reduced effective nuclear charge is the key reason why the outermost electrons are less tightly bound and the atomic radius increases.
The Influence of Principal Quantum Number (n)
The principal quantum number (n) defines the energy level of an electron shell. As you move down a group, the value of 'n' increases. This increase in 'n' directly correlates with the distance of the electron from the nucleus. A higher principal quantum number means a larger, higher-energy orbital, leading to a greater atomic radius. The electrons in higher energy levels are, on average, located farther from the nucleus.
Across a Period: A Contrasting Trend
It's important to contrast the trend down a group with the trend across a period (row) in the periodic table. As you move across a period from left to right, atomic radius generally decreases. This is because the number of protons in the nucleus increases, increasing the effective nuclear charge. This stronger positive charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius. The addition of electrons within the same shell doesn't significantly increase the shielding effect.
Exceptions and Irregularities
While the general trend of increasing atomic radius down a group is robust, there are some minor exceptions and irregularities. These can be attributed to several factors, including:
- Electron-electron repulsion: The repulsion between electrons in the same subshell can slightly expand the atomic radius. This effect is relatively minor compared to the dominant effect of adding electron shells.
- Penetration effects: Electrons in different subshells (s, p, d, f) have different probabilities of being close to the nucleus. Electrons in s orbitals, for instance, penetrate closer to the nucleus than electrons in p orbitals, affecting the shielding and slightly influencing atomic radius.
- Anomalous electron configurations: In some cases, elements exhibit unexpected electron configurations, slightly affecting their atomic radii. These are less common and usually involve transitions metals.
Experimental Evidence and Applications
The increasing atomic radius down a group is not simply a theoretical prediction; it's supported by experimental evidence from various techniques, including X-ray diffraction and spectroscopic methods. This trend has significant implications in various fields:
- Chemistry: Understanding atomic radius is crucial for predicting chemical reactivity, bond lengths, and the properties of compounds. Larger atoms often form weaker bonds.
- Materials Science: The size of atoms directly impacts the properties of materials, influencing their conductivity, strength, and other characteristics. The design of novel materials often relies on understanding atomic radii.
- Physics: Atomic radius is a fundamental parameter in various physical models and calculations, including those used to study the behavior of atoms in different environments and states of matter.
Addressing Common Misconceptions
- Atomic radius is not the same as atomic mass: While atomic mass generally increases down a group (due to increasing numbers of protons and neutrons), it's not directly correlated with atomic radius. The primary factor influencing atomic radius is the number of electron shells and shielding effects.
- The increase in atomic radius is not linear: While the general trend is an increase, the increment in atomic radius between successive elements down a group is not constant. The increase tends to be more significant in the early part of a group and less dramatic later.
- Nuclear charge alone doesn't determine atomic radius: While the increase in nuclear charge contributes, the counteracting effect of increased shielding is far more significant in determining the atomic radius trend.
Conclusion
The increase in atomic radius down a group is a fundamental periodic trend that arises from the interplay of several factors: the addition of electron shells, increased electron shielding, the influence of the principal quantum number, and the resulting reduction in the effective nuclear charge. This trend is supported by experimental evidence and has far-reaching implications across various scientific disciplines. Understanding this concept is essential for grasping the fundamental principles of chemistry and materials science, and for advancing our knowledge of the behavior of matter at the atomic level. By understanding the reasons behind this seemingly simple trend, we unlock a deeper understanding of the intricate workings of the atom and the periodic system. Further research and investigation continuously refine our understanding of this fundamental concept, leading to advancements in numerous scientific fields.
Latest Posts
Latest Posts
-
What Are The Least Common Multiples Of 9 And 12
Mar 19, 2025
-
What Is The Least Common Multiple Of 9 And 7
Mar 19, 2025
-
What Percentage Is 5 Out Of 8
Mar 19, 2025
-
What Is The Least Common Multiple Of 7 And 9
Mar 19, 2025
-
A Diagram Of A Compound Microscope
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about As You Move Down A Group Atomic Radius Increases Because . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
