Are Polar Or Nonpolar Bonds Stronger
Juapaving
Mar 24, 2025 · 5 min read
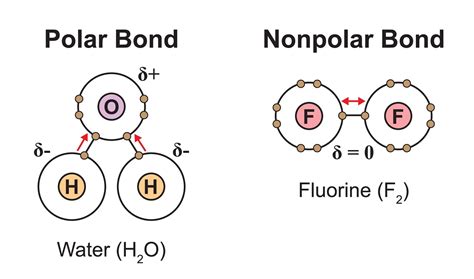
Table of Contents
Are Polar or Nonpolar Bonds Stronger? A Deep Dive into Chemical Bonding
The question of whether polar or nonpolar bonds are stronger is a deceptively complex one. There's no single, universally correct answer, as the strength of a bond depends on several interacting factors. This article will explore the nature of both polar and nonpolar bonds, delve into the forces that govern their strength, and ultimately provide a nuanced understanding of this crucial aspect of chemistry.
Understanding Polar and Nonpolar Bonds: A Foundation
Before comparing bond strengths, let's establish a clear understanding of what differentiates polar and nonpolar bonds. Both types arise from the sharing of electrons between atoms, a phenomenon known as covalent bonding. However, the way electrons are shared dictates the bond's polarity.
Nonpolar Bonds: Equal Sharing
In a nonpolar covalent bond, electrons are shared equally between two atoms. This typically occurs when two atoms of the same element bond (e.g., H₂ , O₂), or when atoms with very similar electronegativities bond (e.g., C-H bonds). Electronegativity, a measure of an atom's ability to attract electrons in a bond, plays a crucial role here. When electronegativity values are nearly identical, the electron cloud is distributed symmetrically between the atoms.
Key Characteristics of Nonpolar Bonds:
- Equal electron sharing: The electrons spend roughly equal time around each nucleus.
- No significant charge separation: There's no significant positive or negative pole within the bond.
- Symmetrical electron distribution: The electron cloud is evenly distributed.
- Examples: H₂, Cl₂, O₂, CH₄
Polar Bonds: Unequal Sharing
In a polar covalent bond, electrons are shared unequally between two atoms. This happens when atoms with significantly different electronegativities bond. The atom with higher electronegativity attracts the shared electrons more strongly, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. This results in a dipole moment, a measure of the bond's polarity.
Key Characteristics of Polar Bonds:
- Unequal electron sharing: The electrons spend more time around the more electronegative atom.
- Significant charge separation: A partial positive and partial negative charge exist within the bond.
- Asymmetrical electron distribution: The electron cloud is shifted towards the more electronegative atom.
- Examples: H₂O (O-H bonds), HCl, NH₃
Factors Affecting Bond Strength: Beyond Polarity
While polarity plays a role, the strength of a bond is primarily determined by other factors:
1. Bond Length: Closer is Stronger
Bond length refers to the average distance between the nuclei of two bonded atoms. Generally, shorter bond lengths correlate with stronger bonds. This is because the closer the nuclei are, the stronger the electrostatic attraction between the positively charged nuclei and the negatively charged electrons.
2. Bond Order: Multiple Bonds are Stronger
Bond order represents the number of electron pairs shared between two atoms. A single bond has a bond order of 1, a double bond has a bond order of 2, and a triple bond has a bond order of 3. Higher bond orders generally indicate stronger bonds. Multiple bonds involve more shared electrons, leading to a stronger electrostatic attraction.
3. Atomic Size: Smaller is Stronger
The sizes of the bonded atoms also influence bond strength. Smaller atoms generally form stronger bonds because the shared electrons are closer to the positively charged nuclei, resulting in a stronger electrostatic attraction.
4. Electronegativity Difference: A Complicating Factor
While a large electronegativity difference leads to a polar bond, the relationship between electronegativity difference and bond strength isn't straightforward. A moderate electronegativity difference can strengthen a bond due to increased electrostatic attraction between the partially charged atoms. However, an extremely large electronegativity difference can lead to an ionic bond, which is fundamentally different from covalent bonds.
Comparing Bond Strengths: A Nuanced Perspective
Now, let's address the central question: Are polar or nonpolar bonds stronger?
The answer is: it depends.
-
In general, nonpolar bonds between atoms of similar size and electronegativity tend to be stronger than polar bonds between atoms of similar size. This is because the equal sharing of electrons in nonpolar bonds leads to a more symmetrical and stable electron distribution.
-
However, the presence of a dipole moment in polar bonds can lead to stronger intermolecular forces. These forces, such as hydrogen bonding and dipole-dipole interactions, influence the overall strength of the substance, even if the individual polar bonds are weaker than comparable nonpolar bonds. Water (H₂O), for example, has relatively weak O-H bonds (polar), but its strong hydrogen bonding leads to a high boiling point and other unique properties.
Consider these examples:
- C-C (nonpolar): A relatively strong bond due to the similar electronegativity and size of carbon atoms.
- C-O (polar): A slightly weaker bond than C-C due to the difference in electronegativity, although still relatively strong.
- O-H (polar): A moderately strong bond, but the strength is significantly influenced by hydrogen bonding in water.
Ionic Bonds: A Separate Category
It's crucial to distinguish covalent bonds from ionic bonds. Ionic bonds involve the transfer of electrons, not the sharing. They form between atoms with significantly different electronegativities (typically a metal and a nonmetal). Ionic bonds are often stronger than even the strongest covalent bonds, primarily due to the strong electrostatic attraction between the oppositely charged ions. However, the comparison isn't entirely fair, as ionic and covalent bonds represent fundamentally different bonding mechanisms.
Conclusion: Context Matters
The question of whether polar or nonpolar bonds are stronger doesn't have a simple yes or no answer. Bond strength is a multifaceted property, determined by bond length, bond order, atomic size, and the degree of electronegativity difference. While nonpolar bonds often exhibit greater inherent strength due to symmetrical electron distribution, polar bonds can lead to stronger intermolecular forces, impacting the overall properties of the substance. Therefore, a comprehensive understanding requires considering all these factors within the specific chemical context. The key takeaway is that the strength of a bond is a nuanced interplay of multiple forces, not solely defined by its polarity.
Latest Posts
Latest Posts
-
Adjectives That Start With A D
Mar 26, 2025
-
Newtons Third Law Real Life Examples
Mar 26, 2025
-
What Is The Part Of Speech Of For
Mar 26, 2025
-
Whats The Square Root Of 125
Mar 26, 2025
-
What Is The Smallest Multiple Of 3 And 4
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Are Polar Or Nonpolar Bonds Stronger . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
