Any Substance That Occupies Space And Has Weight Is ______.
Juapaving
Mar 15, 2025 · 7 min read
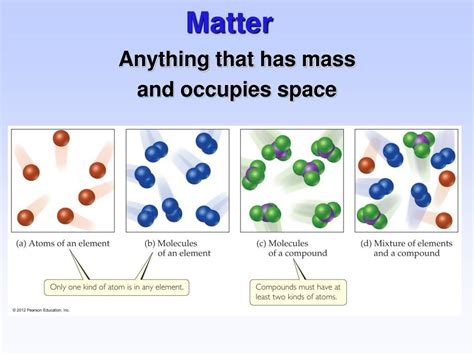
Table of Contents
Any Substance That Occupies Space and Has Weight Is ______: Matter and Its Properties
The answer to the question, "Any substance that occupies space and has weight is ______," is matter. Understanding matter is fundamental to comprehending the physical world around us. From the air we breathe to the stars in the sky, everything is composed of matter. This comprehensive guide will delve into the fascinating world of matter, exploring its properties, classifications, and significance in various fields.
Defining Matter: Space, Weight, and Beyond
Matter, at its simplest, is anything that possesses both mass (a measure of the amount of matter in an object) and volume (the amount of space it occupies). Weight, often confused with mass, is actually the force of gravity acting on an object's mass. While weight can change depending on the gravitational field (you'd weigh less on the moon), the mass remains constant. Therefore, the core definition emphasizes mass and volume rather than weight.
Key Characteristics of Matter:
-
Mass: A measure of the inertia of an object – its resistance to changes in motion. A more massive object requires more force to accelerate than a less massive one. Mass is usually expressed in kilograms (kg) or grams (g).
-
Volume: The three-dimensional space occupied by an object. Volume is typically measured in cubic meters (m³), cubic centimeters (cm³), or liters (L).
-
Weight: The force exerted on an object due to gravity. Weight is dependent on both mass and the gravitational acceleration (g). It's usually expressed in Newtons (N).
-
Inertia: The tendency of an object to resist changes in its state of motion. A stationary object will remain stationary, and a moving object will continue moving at a constant velocity unless acted upon by an external force.
-
Density: The mass per unit volume of a substance. Density helps us understand how tightly packed the particles are within a substance. A high-density object has a lot of mass packed into a small volume.
States of Matter: Solid, Liquid, Gas, and Beyond
Matter exists in various states, each characterized by distinct properties related to the arrangement and movement of its constituent particles (atoms and molecules). The most common states are:
1. Solid:
- Fixed shape and volume: The particles in a solid are closely packed and held together by strong intermolecular forces, resulting in a rigid structure.
- Incompressible: It's difficult to significantly reduce the volume of a solid by applying pressure.
- Low kinetic energy: The particles vibrate in place but don't move freely.
- Examples: Ice, rock, wood, metal.
2. Liquid:
- Fixed volume, but variable shape: Liquids adapt to the shape of their container, but their volume remains relatively constant.
- Slightly compressible: Liquids can be compressed slightly, but not as easily as gases.
- Moderate kinetic energy: Particles move more freely than in solids but are still relatively close together.
- Examples: Water, oil, juice, mercury.
3. Gas:
- Variable shape and volume: Gases expand to fill their container completely.
- Highly compressible: Gases can be easily compressed because their particles are widely spaced.
- High kinetic energy: Particles move rapidly and randomly.
- Examples: Air, oxygen, carbon dioxide, helium.
Beyond the Three Main States:
The three states – solid, liquid, and gas – are the most commonly observed, but matter can exist in other forms under extreme conditions:
-
Plasma: A highly energized state of matter where electrons are stripped from atoms, forming a mixture of ions and free electrons. Plasma is found in stars and lightning.
-
Bose-Einstein Condensate (BEC): A state of matter formed at extremely low temperatures, where a large number of atoms occupy the lowest quantum state.
-
Superfluidity: A state of matter characterized by zero viscosity, meaning it flows without any resistance. Superfluids can climb the walls of containers.
Classifications of Matter: Pure Substances and Mixtures
Matter can also be classified based on its composition:
1. Pure Substances:
A pure substance is matter that has a fixed chemical composition and distinct properties. It cannot be separated into simpler substances by physical methods. Pure substances include:
-
Elements: The simplest form of matter, consisting of only one type of atom. Examples include iron (Fe), oxygen (O₂), and gold (Au).
-
Compounds: Substances formed by the chemical combination of two or more elements in fixed proportions. Examples include water (H₂O), salt (NaCl), and carbon dioxide (CO₂).
2. Mixtures:
A mixture is a combination of two or more substances that are not chemically combined. The components retain their individual properties and can be separated by physical methods. Mixtures can be:
-
Homogeneous: The components are evenly distributed throughout the mixture. Examples include saltwater, air, and sugar dissolved in water.
-
Heterogeneous: The components are not evenly distributed and different phases are visible. Examples include sand and water, oil and water, and a salad.
The Importance of Understanding Matter
Understanding matter is crucial in many fields:
-
Chemistry: Chemistry is the study of matter and its properties, including its composition, structure, and reactions.
-
Physics: Physics explores the fundamental laws governing the behavior of matter and energy.
-
Materials Science: This field investigates the properties of materials and how to design new materials with specific characteristics.
-
Biology: Living organisms are composed of matter, and understanding the properties of biological molecules is essential for comprehending biological processes.
-
Engineering: Engineers use their knowledge of matter to design and build structures, machines, and other technologies.
-
Medicine: The properties of matter are essential for understanding drug delivery, tissue engineering, and medical imaging.
-
Environmental Science: Understanding matter helps us analyze and address environmental problems such as pollution and climate change.
Exploring the Properties of Matter Further: Intensive and Extensive Properties
Matter exhibits various properties that can be used to identify and characterize it. These properties are broadly classified as intensive and extensive properties.
Intensive Properties:
Intensive properties are independent of the amount of matter present. They are inherent characteristics of the substance itself. Examples include:
- Density: Mass per unit volume.
- Melting point: The temperature at which a solid turns into a liquid.
- Boiling point: The temperature at which a liquid turns into a gas.
- Color: The visual appearance of the substance.
- Odor: The smell of the substance.
- Hardness: Resistance to scratching or indentation.
- Conductivity: Ability to conduct heat or electricity.
Extensive Properties:
Extensive properties depend on the amount of matter present. They are additive and change proportionally with the size of the sample. Examples include:
- Mass: The amount of matter in a sample.
- Volume: The space occupied by a sample.
- Length: The distance between two points on an object.
- Heat capacity: The amount of heat required to raise the temperature of a sample by a certain amount.
Understanding the difference between intensive and extensive properties is important for characterizing substances and solving problems related to chemical and physical changes.
Changes in Matter: Physical and Chemical Changes
Matter can undergo two main types of changes:
1. Physical Changes:
Physical changes alter the form or appearance of matter but do not change its chemical composition. The substance remains the same chemically. Examples include:
- Changes of state: Melting, freezing, boiling, condensation, sublimation, and deposition.
- Dissolving: A substance dissolves in a solvent, forming a solution.
- Cutting: Dividing a substance into smaller pieces.
- Crushing: Reducing the size of a substance.
- Shape changes: Bending, stretching, or reshaping a substance.
2. Chemical Changes:
Chemical changes, also known as chemical reactions, involve a change in the chemical composition of matter. New substances with different properties are formed. Examples include:
- Burning: Combustion reactions, where a substance reacts with oxygen, releasing heat and light.
- Rusting: Oxidation of iron, forming iron oxide.
- Cooking: Chemical changes occur when food is cooked, altering its taste, texture, and nutritional value.
- Digestion: Chemical breakdown of food in the body.
- Photosynthesis: Plants convert light energy into chemical energy, producing glucose and oxygen.
Conclusion: The Ever-Expanding World of Matter
In conclusion, any substance that occupies space and has weight is matter. This seemingly simple definition opens a gateway to a vast and fascinating world of scientific exploration. Understanding the properties, states, and classifications of matter is fundamental to comprehending the universe and the technologies that shape our lives. From the smallest atoms to the largest galaxies, matter underlies everything we experience. This detailed exploration provides a solid foundation for further delving into the complexities and wonders of the material world. Continued learning and investigation will continue to reveal even more about the intricacies and importance of matter in our universe.
Latest Posts
Latest Posts
-
What Is An Operator In Biology
Mar 17, 2025
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Any Substance That Occupies Space And Has Weight Is ______. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
