An Atom Gets An Overall Positive Charge By
Juapaving
Mar 28, 2025 · 5 min read
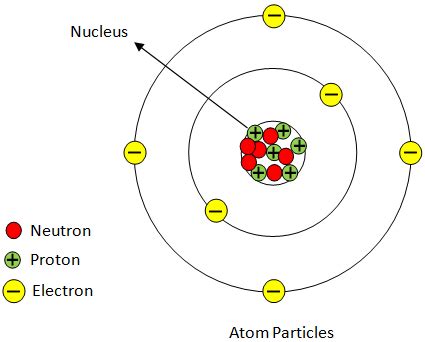
Table of Contents
An Atom Gets an Overall Positive Charge By: Ionization and Beyond
Atoms, the fundamental building blocks of matter, are usually electrically neutral. This neutrality stems from the equal number of positively charged protons in the nucleus and negatively charged electrons orbiting around it. However, atoms can gain or lose electrons, leading to a net positive or negative charge. This process is crucial in many chemical and physical phenomena, from the formation of ionic bonds to the operation of electronic devices. This article delves deep into the mechanisms by which an atom achieves an overall positive charge, exploring the underlying principles and diverse applications.
The Primary Mechanism: Ionization
The most common way an atom acquires a positive charge is through ionization. Ionization is the process where an atom loses one or more electrons, resulting in an imbalance between the number of protons and electrons. Since protons carry a positive charge and electrons carry a negative charge, the loss of negatively charged electrons leaves the atom with a net positive charge. This positively charged atom is called a cation.
Mechanisms of Ionization
Several factors can trigger ionization:
-
High Temperatures: At extremely high temperatures, the kinetic energy of atoms increases significantly. This increased energy can overcome the electrostatic attraction between the nucleus and the outermost electrons, causing electrons to be ejected. This is commonly observed in plasmas, like those found in stars and fluorescent lights.
-
Radiation: Exposure to high-energy radiation, such as X-rays or gamma rays, can ionize atoms. The high-energy photons can interact with electrons, transferring enough energy to overcome the binding energy and eject the electron. This principle is utilized in various technologies, including medical imaging (X-rays) and radiation therapy.
-
Electron Impact: Collisions with high-velocity electrons can also ionize atoms. The kinetic energy of the colliding electron is transferred to an atomic electron, potentially ejecting it from the atom. This mechanism is prevalent in gas discharge tubes and particle accelerators.
-
Chemical Reactions: During certain chemical reactions, atoms can lose electrons to other atoms with higher electronegativity. This electron transfer creates ions, with the atom losing electrons becoming a cation. This is a fundamental aspect of ionic bonding, where oppositely charged ions attract each other to form stable compounds.
Factors Influencing Ionization Energy
The ease with which an atom can be ionized is determined by its ionization energy. Ionization energy is the minimum energy required to remove an electron from a neutral gaseous atom in its ground state. Several factors influence ionization energy:
-
Nuclear Charge: A higher nuclear charge (more protons) increases the electrostatic attraction between the nucleus and electrons, making it harder to remove an electron and thus increasing the ionization energy.
-
Atomic Radius: Larger atoms have their outermost electrons farther from the nucleus, experiencing weaker electrostatic attraction. This results in lower ionization energy.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outer electrons, leading to lower ionization energy.
-
Electron Configuration: Electrons in filled subshells are more stable than those in partially filled subshells. Removing an electron from a filled subshell requires more energy than removing one from a partially filled subshell.
Types of Ions and Multiple Ionization
Atoms can lose multiple electrons, resulting in cations with multiple positive charges. For example, a calcium atom (Ca) can lose two electrons to become a Ca²⁺ ion (calcium ion with a +2 charge). The energy required to remove successive electrons increases progressively, as the remaining electrons are more strongly bound to the increasingly positive ion.
Beyond Ionization: Other Ways to Achieve a Positive Charge
While ionization is the most common mechanism, other less frequent processes can also contribute to an atom having an overall positive charge:
-
Beta Decay: This radioactive decay process involves the transformation of a neutron into a proton, an electron (beta particle), and an antineutrino. The resulting atom has one more proton, leading to a net increase in positive charge.
-
Proton Transfer: In certain chemical reactions, a proton (H⁺ ion) can be transferred from one atom to another. The atom that loses the proton gains a net negative charge while the recipient gains a net positive charge.
Applications of Positively Charged Atoms
Positively charged atoms, or cations, play vital roles in numerous scientific and technological applications:
-
Ionic Compounds: Cations form the basis of ionic compounds, where they are electrostatically attracted to anions (negatively charged ions) to create stable, electrically neutral compounds. These compounds are essential in various applications, including fertilizers, pharmaceuticals, and construction materials.
-
Electrochemistry: Cations participate in electrochemical processes, such as batteries and fuel cells. The movement of cations through electrolytes enables the generation and storage of electrical energy.
-
Mass Spectrometry: Mass spectrometry techniques utilize the charge-to-mass ratio of ions to identify and quantify different atoms and molecules. The creation of positively charged ions is crucial in this analytical technique.
-
Plasma Physics: Plasmas, which are highly ionized gases, are used in various technologies, including plasma displays, plasma etching in semiconductor manufacturing, and fusion research. The positive ions in plasmas contribute significantly to their unique properties.
-
Medical Imaging and Therapy: As mentioned earlier, radiation can ionize atoms, and this principle is utilized in medical imaging techniques like X-rays and CT scans. Furthermore, radiation therapy employs ionizing radiation to damage cancer cells.
Conclusion
The acquisition of a positive charge by an atom, predominantly through ionization, is a fundamental process in chemistry and physics. Understanding the mechanisms of ionization and the factors that influence ionization energy is crucial for interpreting and manipulating the behavior of matter at the atomic level. The applications of positively charged atoms are vast and far-reaching, spanning across various scientific disciplines and technological advancements. From the formation of ionic compounds to advanced technologies like plasma displays and medical imaging, the role of positively charged atoms is undeniable and continues to expand as our understanding of atomic-level processes grows. The ongoing research in areas like plasma physics and materials science further underscores the significant importance of this fundamental concept. The ability to control and manipulate the ionization of atoms holds the key to unlocking future technological breakthroughs and advancements across diverse fields.
Latest Posts
Latest Posts
-
5 Levels Of Organization In An Ecosystem
Mar 31, 2025
-
Would Silver React With Dilute Sulfuric Acid
Mar 31, 2025
-
How Many Factors Does A Composite Number Have
Mar 31, 2025
-
What Is 4 The Square Root Of
Mar 31, 2025
-
Tendons And Ligaments Are Examples Of
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about An Atom Gets An Overall Positive Charge By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
