Acetic Acid Vs Glacial Acetic Acid
Juapaving
Mar 24, 2025 · 5 min read
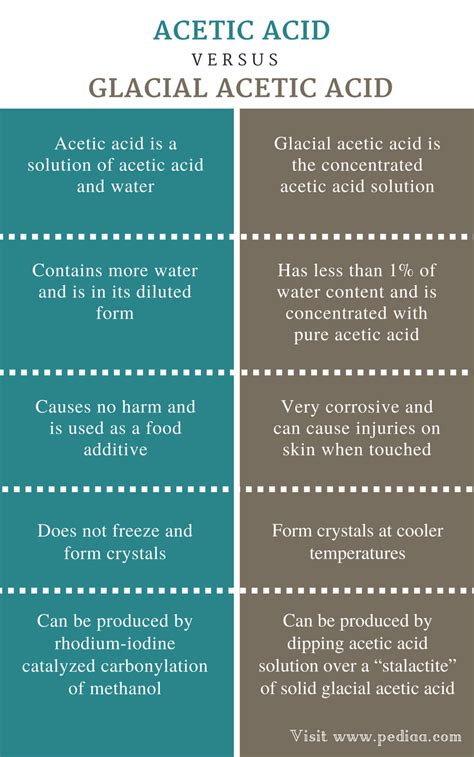
Table of Contents
Acetic Acid vs. Glacial Acetic Acid: Understanding the Differences
Acetic acid, a ubiquitous organic compound, is found in various forms, most notably as vinegar and in its pure, concentrated form known as glacial acetic acid. While both share the same chemical formula (CH₃COOH), their properties, applications, and handling differ significantly. Understanding these differences is crucial for anyone working with this versatile chemical. This article delves deep into the nuances of acetic acid versus glacial acetic acid, exploring their properties, uses, safety precautions, and more.
What is Acetic Acid?
Acetic acid is a weak organic acid with a pungent, vinegar-like odor. It’s the main component of vinegar, typically making up around 4-7% by volume. The remainder of vinegar consists of water and trace amounts of other compounds that contribute to its flavor and aroma. While vinegar is the most common household form, acetic acid also occurs naturally in various fruits, vegetables, and as a byproduct of fermentation.
Properties of Acetic Acid (Dilute):
- Weak Acid: It only partially dissociates in water, meaning it doesn't release all its hydrogen ions (H⁺). This makes it less corrosive than strong acids like hydrochloric acid.
- Water-Miscible: It readily dissolves in water, making it easy to dilute.
- Low Concentration: In vinegar and other diluted forms, the concentration of acetic acid is relatively low.
- Mild Odor: The odor is noticeable but not overpowering in dilute solutions.
- Versatile Uses: Due to its mild acidity and water solubility, it finds applications in cooking, cleaning, and as a preservative.
What is Glacial Acetic Acid?
Glacial acetic acid is the anhydrous (water-free) form of acetic acid. It's called "glacial" because it solidifies into ice-like crystals at slightly below room temperature (16.7°C or 62°F). This high purity and concentration give it very different characteristics compared to the dilute acetic acid found in vinegar.
Properties of Glacial Acetic Acid:
- Stronger Acid (Concentrated): While still a weak acid in terms of its dissociation constant, its high concentration makes it significantly more acidic and corrosive than dilute acetic acid.
- Anhydrous: The absence of water drastically alters its properties and reactivity.
- High Concentration: Typically, glacial acetic acid is at least 99% pure acetic acid.
- Corrosive: Prolonged contact with skin or eyes can cause severe burns and irritation. Inhaling its vapor can irritate the respiratory system.
- Freezing Point: Its freezing point is relatively high (16.7°C), which is why it often appears as ice-like crystals.
- Specific Uses: Its high purity makes it a crucial reagent in various chemical syntheses, industrial processes, and laboratory settings.
Key Differences: Acetic Acid vs. Glacial Acetic Acid
The table below summarizes the key differences between acetic acid (as typically found in vinegar) and glacial acetic acid:
| Feature | Acetic Acid (Dilute) | Glacial Acetic Acid |
|---|---|---|
| Water Content | High | Virtually None |
| Concentration | Low (4-7% in vinegar) | High (99% or more) |
| Acidity | Mild | Strong (concentrated) |
| Corrosiveness | Low | High |
| Odor | Mild, vinegar-like | Pungent, strong |
| Freezing Point | Well below 0°C | 16.7°C (62°F) |
| Applications | Food, cleaning, etc. | Chemical synthesis, industry |
| Safety | Relatively safe | Requires careful handling |
Applications of Acetic Acid (Dilute and Concentrated):
Both dilute and glacial acetic acid find diverse applications across multiple industries:
Dilute Acetic Acid (Vinegar):
- Food Industry: Vinegar is used as a condiment, preservative, and marinade. Its acidity inhibits bacterial growth, extending the shelf life of food.
- Household Cleaning: It’s a natural and effective cleaning agent, removing stains and disinfecting surfaces.
- Cosmetics: It's sometimes used in hair products and toners for its mild acidity.
- Pickling: Vinegar is essential in preserving vegetables and other foods through pickling.
Glacial Acetic Acid:
- Chemical Industry: A key reagent in the production of vinyl acetate monomer (VAM), used to make plastics, adhesives, and paints. It's also used in the manufacture of cellulose acetate, a component of textiles and photographic film.
- Pharmaceutical Industry: Used as a solvent and reagent in the synthesis of various pharmaceuticals.
- Laboratory Applications: A common solvent and reactant in various chemical experiments and analyses.
- Textile Industry: Used in dyeing and finishing processes.
- Food Industry (Specific Applications): While dilute acetic acid is common, glacial acetic acid finds niche uses in specific food processing applications requiring high purity and concentration.
Safety Precautions:
Handling both forms of acetic acid requires appropriate safety measures, but the precautions are significantly stricter for glacial acetic acid due to its higher corrosiveness.
Handling Dilute Acetic Acid (Vinegar):
- Eye Protection: Although less hazardous, it’s always good practice to wear eye protection to prevent accidental splashes.
- Ventilation: Good ventilation is recommended, especially when dealing with larger quantities.
- Skin Contact: Wash any spills on skin immediately with water.
Handling Glacial Acetic Acid:
- Personal Protective Equipment (PPE): This includes safety glasses, gloves (chemical-resistant), and a lab coat. A face shield is strongly recommended for added protection.
- Fume Hood: All handling should occur under a well-ventilated fume hood to prevent inhalation of vapors.
- Spill Response: Have a spill kit readily available. Neutralize spills with a suitable base, such as sodium bicarbonate (baking soda), followed by thorough cleaning and disposal according to regulations.
- Storage: Store in a cool, dry place away from incompatible materials.
- Emergency Procedures: Be aware of emergency procedures in case of spills or accidental exposure.
Environmental Considerations:
Both forms of acetic acid are relatively biodegradable and have a low environmental impact when properly handled and disposed of. However, large spills or improper disposal can negatively affect aquatic life. Always follow local regulations for the proper disposal of acetic acid waste.
Conclusion:
Acetic acid, in its various forms, is a versatile and important chemical with numerous applications. Understanding the differences between dilute acetic acid (as found in vinegar) and glacial acetic acid, particularly their differing levels of acidity, corrosiveness, and required safety precautions, is critical for safe and effective handling. While vinegar is a relatively safe household item, glacial acetic acid demands respect and careful handling to prevent accidents and injury. Always prioritize safety and adhere to appropriate protocols when working with this powerful chemical. Choosing the right form of acetic acid depends entirely on the application, with the purity and concentration requirements dictating the appropriate choice. This detailed comparison highlights the importance of understanding these key differences to ensure safe and efficient utilization in various industrial and laboratory settings.
Latest Posts
Latest Posts
-
Lcm Of 6 8 And 12
Mar 26, 2025
-
The Minimum Wage Is An Example Of A
Mar 26, 2025
-
Word That Starts With A V
Mar 26, 2025
-
The Red Data Book Keeps A Record Of All The
Mar 26, 2025
-
What Are The Inner Transition Metals
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Acetic Acid Vs Glacial Acetic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
